You are browsing environment: FUNGIDB
CAZyme Information: FPRO_15501-t41_1-p1
You are here: Home > Sequence: FPRO_15501-t41_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Fusarium proliferatum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Nectriaceae; Fusarium; Fusarium proliferatum | |||||||||||
| CAZyme ID | FPRO_15501-t41_1-p1 | |||||||||||
| CAZy Family | GT34 | |||||||||||
| CAZyme Description | uncharacterized protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE5 | 68 | 180 | 3.9e-21 | 0.6243386243386243 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395860 | Cutinase | 1.43e-24 | 68 | 180 | 1 | 110 | Cutinase. |
| 238382 | Lipase | 0.006 | 138 | 162 | 17 | 41 | Lipase. Lipases are esterases that can hydrolyze long-chain acyl-triglycerides into di- and monoglycerides, glycerol, and free fatty acids at a water/lipid interface. A typical feature of lipases is "interfacial activation", the process of becoming active at the lipid/water interface, although several examples of lipases have been identified that do not undergo interfacial activation . The active site of a lipase contains a catalytic triad consisting of Ser - His - Asp/Glu, but unlike most serine proteases, the active site is buried inside the structure. A "lid" or "flap" covers the active site, making it inaccessible to solvent and substrates. The lid opens during the process of interfacial activation, allowing the lipid substrate access to the active site. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 9.35e-118 | 1 | 181 | 1 | 181 | |
| 9.35e-118 | 1 | 181 | 1 | 181 | |
| 9.35e-118 | 1 | 181 | 1 | 181 | |
| 5.79e-69 | 1 | 180 | 1 | 183 | |
| 2.46e-44 | 1 | 180 | 1 | 187 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.20e-15 | 64 | 180 | 74 | 187 | Structure of cutinase from Trichoderma reesei in its native form. [Trichoderma reesei QM6a],4PSD_A Structure of Trichoderma reesei cutinase native form. [Trichoderma reesei QM6a],4PSE_A Trichoderma reesei cutinase in complex with a C11Y4 phosphonate inhibitor [Trichoderma reesei QM6a],4PSE_B Trichoderma reesei cutinase in complex with a C11Y4 phosphonate inhibitor [Trichoderma reesei QM6a] |
|
| 1.41e-11 | 61 | 178 | 14 | 131 | Crystal structure of Aspergillus oryzae cutinase [Aspergillus oryzae] |
|
| 6.23e-11 | 61 | 178 | 5 | 122 | Structure of Aspergillus oryzae cutinase expressed in Pichia pastoris, crystallized in the presence of Paraoxon [Aspergillus oryzae] |
|
| 8.55e-10 | 59 | 179 | 19 | 142 | Chain A, CUTINASE [Fusarium vanettenii] |
|
| 2.46e-08 | 61 | 164 | 16 | 119 | Chain A, cutinase [Malbranchea cinnamomea] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.29e-21 | 59 | 181 | 31 | 152 | Cutinase CUT2 OS=Magnaporthe oryzae (strain 70-15 / ATCC MYA-4617 / FGSC 8958) OX=242507 GN=CUT2 PE=1 SV=1 |
|
| 2.01e-21 | 7 | 180 | 11 | 177 | Cutinase OS=Blumeria hordei OX=2867405 GN=CUT1 PE=3 SV=1 |
|
| 3.37e-18 | 69 | 178 | 31 | 138 | Cutinase OS=Botryotinia fuckeliana OX=40559 GN=cutA PE=1 SV=1 |
|
| 3.85e-16 | 59 | 171 | 50 | 158 | Cutinase 4 OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=cut4 PE=2 SV=1 |
|
| 9.95e-16 | 69 | 180 | 31 | 139 | Cutinase OS=Monilinia fructicola OX=38448 GN=CUT1 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
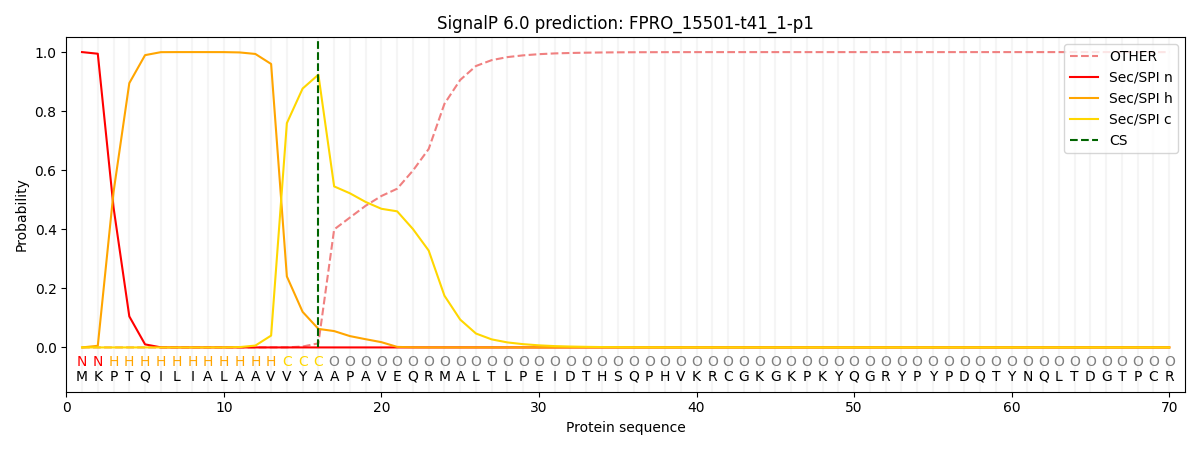
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000287 | 0.999715 | CS pos: 16-17. Pr: 0.9235 |
