You are browsing environment: FUNGIDB
CAZyme Information: FPRO_12622-t41_1-p1
You are here: Home > Sequence: FPRO_12622-t41_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Fusarium proliferatum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Nectriaceae; Fusarium; Fusarium proliferatum | |||||||||||
| CAZyme ID | FPRO_12622-t41_1-p1 | |||||||||||
| CAZy Family | GH47 | |||||||||||
| CAZyme Description | probable polysaccharide deacetylase family protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 45 | 149 | 1e-17 | 0.7923076923076923 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 200563 | CE4_HpPgdA_like | 3.28e-117 | 8 | 272 | 2 | 258 | Catalytic domain of Helicobacter pylori peptidoglycan deacetylase (HpPgdA) and similar proteins. This family is represented by a peptidoglycan deacetylase (HP0310, HpPgdA) from the gram-negative pathogen Helicobacter pylori. HpPgdA has the ability to bind a metal ion at the active site and is responsible for a peptidoglycan modification that counteracts the host immune response. It functions as a homotetramer. The monomer is composed of a 7-stranded barrel with detectable sequence similarity to the 6-stranded barrel NodB homology domain of polysaccharide deacetylase (DCA)-like proteins in the CE4 superfamily, which removes N-linked or O-linked acetyl groups from cell wall polysaccharides. In contrast to typical NodB-like DCAs, HpPgdA does not exhibit a solvent-accessible polysaccharide binding groove, suggesting that the enzyme binds a small molecule at the active site. |
| 213021 | CE4_PuuE_HpPgdA_like | 8.12e-59 | 8 | 270 | 2 | 247 | Catalytic domain of bacterial PuuE allantoinases, Helicobacter pylori peptidoglycan deacetylase (HpPgdA), and similar proteins. This family is a member of the very large and functionally diverse carbohydrate esterase 4 (CE4) superfamily. It contains bacterial PuuE (purine utilization E) allantoinases, a peptidoglycan deacetylase from Helicobacter pylori (HpPgdA), Escherichia coli ArnD, and many uncharacterized homologs from all three kingdoms of life. PuuE allantoinase appears to be metal-independent and specifically catalyzes the hydrolysis of (S)-allantoin into allantoic acid. Different from PuuE allantoinase, HpPgdA has the ability to bind a metal ion at the active site and is responsible for a peptidoglycan modification that counteracts the host immune response. Both PuuE allantoinase and HpPgdA function as a homotetramer. The monomer is composed of a 7-stranded barrel with detectable sequence similarity to the 6-stranded barrel NodB homology domain of polysaccharide deacetylase (DCA)-like proteins in the CE4 superfamily, which removes N-linked or O-linked acetyl groups from cell wall polysaccharides. However, in contrast with the typical DCAs, PuuE allantoinase and HpPgdA might not exhibit a solvent-accessible polysaccharide binding groove and only recognize a small substrate molecule. ArnD catalyzes the deformylation of 4-deoxy-4-formamido-L-arabinose-phosphoundecaprenol to 4-amino-4-deoxy-L-arabinose-phosphoundecaprenol. |
| 200601 | CE4_PuuE_like | 1.96e-30 | 42 | 274 | 63 | 281 | Putative catalytic domain of uncharacterized prokaryotic polysaccharide deacetylases similar to bacterial PuuE allantoinases. The family includes a group of uncharacterized prokaryotic polysaccharide deacetylases (DCAs) that show high sequence similarity to the catalytic domain of bacterial PuuE (purine utilization E) allantoinases. PuuE allantoinase specifically catalyzes the hydrolysis of (S)-allantoin into allantoic acid. It functions as a homotetramer. Its monomer is composed of a 7-stranded barrel with detectable sequence similarity to the 6-stranded barrel NodB homology domain of DCA-like proteins in the CE4 superfamily, which removes N-linked or O-linked acetyl groups from cell wall polysaccharides. PuuE allantoinase appears to be metal-independent and acts on a small substrate molecule, which is distinct from the common feature of DCAs which are normally metal ion dependent and recognize multimeric substrates. |
| 223798 | CDA1 | 1.22e-21 | 43 | 271 | 79 | 260 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| 200566 | CE4_PuuE_HpPgdA_like_2 | 5.51e-20 | 43 | 151 | 33 | 141 | Putative catalytic domain of uncharacterized prokaryotic polysaccharide deacetylases similar to bacterial PuuE allantoinases and Helicobacter pylori peptidoglycan deacetylase (HpPgdA). This family contains many uncharacterized prokaryotic polysaccharide deacetylases (DCAs) that show high sequence similarity to the catalytic domain of bacterial PuuE allantoinases and Helicobacter pylori peptidoglycan deacetylase (HpPgdA). PuuE allantoinase appears to be metal-independent and specifically catalyzes the hydrolysis of (S)-allantoin into allantoic acid. Different from PuuE allantoinase, HpPgdA has the ability to bind a metal ion at the active site and is responsible for a peptidoglycan modification that counteracts the host immune response. Both PuuE allantoinase and HpPgdA function as homotetramers. The monomer is composed of a 7-stranded barrel with detectable sequence similarity to the 6-stranded barrel NodB homology domain of DCA-like proteins in the CE4 superfamily, which removes N-linked or O-linked acetyl groups from cell wall polysaccharides. In contrast to typical NodB-like DCAs, PuuE allantoinase and HpPgdA do not exhibit a solvent-accessible polysaccharide binding groove and might only bind a small molecule at the active site. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.56e-20 | 8 | 268 | 18 | 267 | |
| 4.13e-15 | 43 | 277 | 62 | 283 | |
| 1.58e-14 | 31 | 277 | 57 | 290 | |
| 2.16e-14 | 31 | 277 | 57 | 290 | |
| 3.95e-14 | 31 | 277 | 55 | 288 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.16e-130 | 4 | 276 | 36 | 323 | Crystal structure of putative peptidoglycan deactelyase (HP0310) from Helicobacter pylori [Helicobacter pylori G27],3QBU_B Crystal structure of putative peptidoglycan deactelyase (HP0310) from Helicobacter pylori [Helicobacter pylori G27],3QBU_C Crystal structure of putative peptidoglycan deactelyase (HP0310) from Helicobacter pylori [Helicobacter pylori G27],3QBU_D Crystal structure of putative peptidoglycan deactelyase (HP0310) from Helicobacter pylori [Helicobacter pylori G27],4LY4_A Crystal structure of peptidoglycan deacetylase (HP0310) with Zinc from Helicobacter pylori [Helicobacter pylori G27],4LY4_B Crystal structure of peptidoglycan deacetylase (HP0310) with Zinc from Helicobacter pylori [Helicobacter pylori G27],4LY4_C Crystal structure of peptidoglycan deacetylase (HP0310) with Zinc from Helicobacter pylori [Helicobacter pylori G27],4LY4_D Crystal structure of peptidoglycan deacetylase (HP0310) with Zinc from Helicobacter pylori [Helicobacter pylori G27] |
|
| 1.66e-19 | 4 | 240 | 23 | 249 | Crystal structure of putative polysaccharide deacetylase from Mycobacterium smegmatis [Mycolicibacterium smegmatis MC2 155],3RXZ_B Crystal structure of putative polysaccharide deacetylase from Mycobacterium smegmatis [Mycolicibacterium smegmatis MC2 155],3RXZ_C Crystal structure of putative polysaccharide deacetylase from Mycobacterium smegmatis [Mycolicibacterium smegmatis MC2 155],3RXZ_D Crystal structure of putative polysaccharide deacetylase from Mycobacterium smegmatis [Mycolicibacterium smegmatis MC2 155] |
|
| 1.37e-08 | 44 | 133 | 36 | 125 | Crystal structure of the Bc1960 peptidoglycan N-acetylglucosamine deacetylase in complex with 4-naphthalen-1-yl-~{N}-oxidanyl-benzamide [Bacillus cereus ATCC 14579] |
|
| 3.40e-08 | 44 | 122 | 36 | 114 | Crystal structure of the Bc1960 peptidoglycan N-acetylglucosamine deacetylase in complex with 4-naphthalen-1-yl-~{N}-oxidanyl-benzamide [Bacillus cereus ATCC 14579],5O6Y_C Crystal structure of the Bc1960 peptidoglycan N-acetylglucosamine deacetylase in complex with 4-naphthalen-1-yl-~{N}-oxidanyl-benzamide [Bacillus cereus ATCC 14579],5O6Y_D Crystal structure of the Bc1960 peptidoglycan N-acetylglucosamine deacetylase in complex with 4-naphthalen-1-yl-~{N}-oxidanyl-benzamide [Bacillus cereus ATCC 14579] |
|
| 3.93e-08 | 43 | 276 | 87 | 310 | Crystal structure of a Polysaccharide deacetylase family protein from Burkholderia pseudomallei [Burkholderia pseudomallei],3S6O_B Crystal structure of a Polysaccharide deacetylase family protein from Burkholderia pseudomallei [Burkholderia pseudomallei],3S6O_C Crystal structure of a Polysaccharide deacetylase family protein from Burkholderia pseudomallei [Burkholderia pseudomallei],3S6O_D Crystal structure of a Polysaccharide deacetylase family protein from Burkholderia pseudomallei [Burkholderia pseudomallei] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.55e-130 | 4 | 276 | 3 | 290 | Peptidoglycan deacetylase OS=Helicobacter pylori (strain G27) OX=563041 GN=pgdA PE=1 SV=1 |
|
| 1.17e-128 | 4 | 276 | 3 | 290 | Peptidoglycan deacetylase OS=Helicobacter pylori (strain ATCC 700392 / 26695) OX=85962 GN=pgdA PE=1 SV=1 |
|
| 7.88e-59 | 3 | 278 | 37 | 337 | Peptidoglycan deacetylase-like protein FGM2 OS=Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) OX=229533 GN=FGM2 PE=2 SV=1 |
|
| 1.20e-07 | 44 | 133 | 96 | 185 | Peptidoglycan-N-acetylglucosamine deacetylase BC_1960 OS=Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG 7414 / JCM 2152 / NBRC 15305 / NCIMB 9373 / NCTC 2599 / NRRL B-3711) OX=226900 GN=BC_1960 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
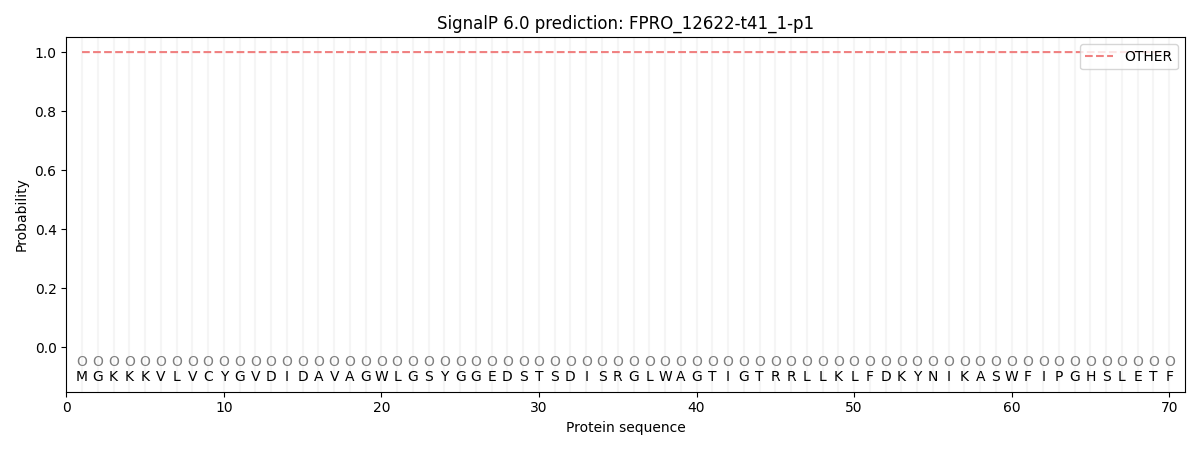
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000054 | 0.000000 |
