You are browsing environment: FUNGIDB
CAZyme Information: FPRN_04030-t42_1-p1
You are here: Home > Sequence: FPRN_04030-t42_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Fusarium proliferatum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Nectriaceae; Fusarium; Fusarium proliferatum | |||||||||||
| CAZyme ID | FPRN_04030-t42_1-p1 | |||||||||||
| CAZy Family | PL3 | |||||||||||
| CAZyme Description | uncharacterized protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 412051 | CdiI_ECL-like | 0.001 | 181 | 269 | 36 | 120 | inhibitor (or immunity protein) of the contact-dependent growth inhibition (CDI) system of Enterobacter cloacae, and similar proteins. CDI toxins are expressed by gram-negative bacteria as part of a mechanism to inhibit the growth of neighboring cells. This model represents the inhibitor (CdiI, also called CdiI immunity protein) of the CdiA effector protein from Enterobacter cloacae, and similar proteins. CdiA secretion is dependent on the outer membrane protein CdiB. Upon binding to a receptor on the surface of target bacteria, the CDI toxin is delivered via its C-terminal domain (CdiA-CT). The CdiI immunity proteins are intracellular proteins that inactivate the toxin/effector protein to prevent auto-inhibition. They are specific for their cognate CdiA-CT and do not protect cells from the toxins of other CDI+ bacteria. Thus, CDI systems encode a complex network of toxin-immunity protein pairs that are deployed for intercellular competition. Although E. cloacae CdiA-CT has structural homology to the C-terminal nuclease domain of colicin E3, which cleaves 16S ribosomal RNA to disrupt protein synthesis, and has been shown to use the same nuclease activity to inhibit bacterial growth, the corresponding CdiI immunity proteins are unrelated in sequence, structure and toxin-binding sites. Structural homology searches reveal that E. cloacae CdiI is most similar to the Whirly family of single-stranded DNA-binding proteins. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 467 | 1 | 470 | |
| 0.0 | 1 | 467 | 1 | 490 | |
| 0.0 | 1 | 467 | 1 | 490 | |
| 0.0 | 1 | 467 | 1 | 490 | |
| 1.93e-300 | 1 | 467 | 1 | 462 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.14e-07 | 140 | 301 | 114 | 271 | Structure of N2152 from Neocallimastix frontalis [Neocallimastix frontalis] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
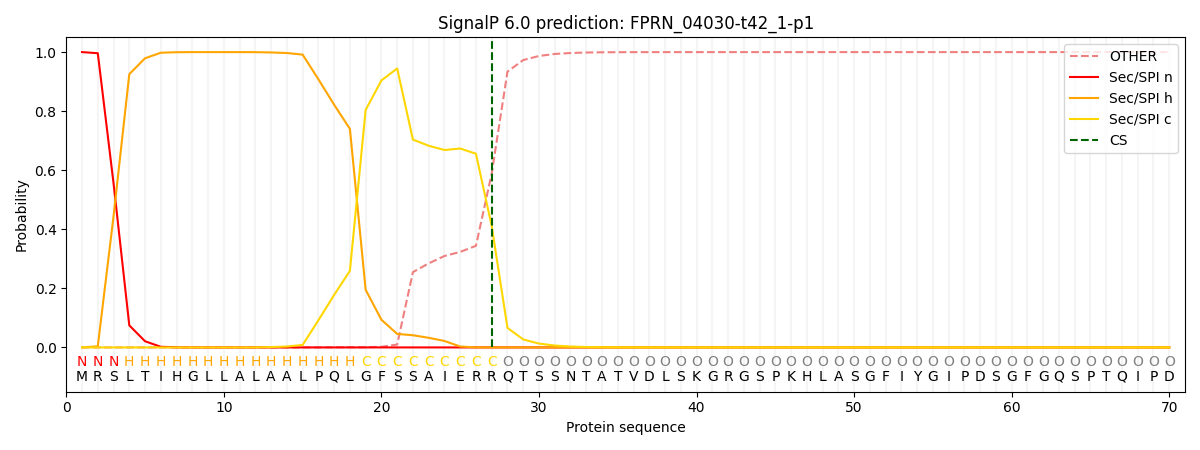
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000234 | 0.999744 | CS pos: 27-28. Pr: 0.4110 |
