You are browsing environment: FUNGIDB
CAZyme Information: EPrPVT00000018749-p1
You are here: Home > Sequence: EPrPVT00000018749-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
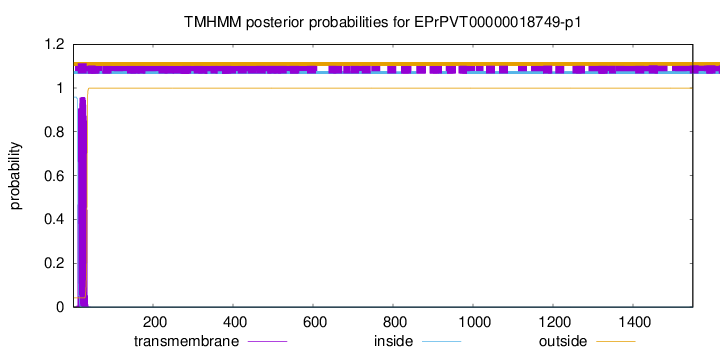
TMHMM annotations
Basic Information help
| Species | Phytopythium vexans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Pythiaceae; Phytopythium; Phytopythium vexans | |||||||||||
| CAZyme ID | EPrPVT00000018749-p1 | |||||||||||
| CAZy Family | GH3 | |||||||||||
| CAZyme Description | Serine protease | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT71 | 1186 | 1363 | 1.6e-37 | 0.7765151515151515 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173791 | Peptidases_S8_Kp43_protease | 1.90e-82 | 395 | 709 | 1 | 293 | Peptidase S8 family domain in Kp43 proteases. Kp43 proteases are members of the peptidase S8 or Subtilase clan of proteases. They have an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure (an example of convergent evolution). Kp43 is topologically similar to kexin and furin both of which are proprotein convertases, but differ in amino acids sequence and the position of its C-terminal barrel. Kp43 has 3 Ca2+ binding sites that differ from the corresponding sites in the other known subtilisin-like proteases. KP-43 protease is known to be an oxidation-resistant protease when compared with the other subtilisin-like proteases |
| 395035 | Peptidase_S8 | 1.59e-42 | 400 | 711 | 1 | 275 | Subtilase family. Subtilases are a family of serine proteases. They appear to have independently and convergently evolved an Asp/Ser/His catalytic triad, like that found in the trypsin serine proteases (see pfam00089). Structure is an alpha/beta fold containing a 7-stranded parallel beta sheet, order 2314567. |
| 173812 | Peptidases_S8_1 | 2.83e-37 | 400 | 674 | 1 | 245 | Peptidase S8 family domain, uncharacterized subfamily 1. This family is a member of the Peptidases S8 or Subtilases serine endo- and exo-peptidase clan. They have an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. The stability of subtilases may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. Some members of this clan contain disulfide bonds. These enzymes can be intra- and extracellular, some function at extreme temperatures and pH values. |
| 402574 | Mannosyl_trans3 | 3.97e-30 | 1186 | 1384 | 2 | 273 | Mannosyltransferase putative. This family is conserved in fungi. Several members are annotated as being alpha-1,3-mannosyltransferase but this could not be confirmed. |
| 173801 | Peptidases_S8_C5a_Peptidase | 5.56e-29 | 397 | 678 | 7 | 294 | Peptidase S8 family domain in Streptococcal C5a peptidases. Streptococcal C5a peptidase (SCP), is a highly specific protease and adhesin/invasin. The subtilisin-like protease domain is located at the N-terminus and contains a protease-associated domain inserted into a loop. There are three fibronectin type III (Fn) domains at the C-terminus. SCP binds to integrins with the help of Arg-Gly-Asp motifs which are thought to stabilize conformational changes required for substrate binding. Peptidases S8 or Subtilases are a serine endo- and exo-peptidase clan. They have an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. The stability of subtilases may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. Some members of this clan contain disulfide bonds. These enzymes can be intra- and extracellular, some function at extreme temperatures and pH values. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.45e-87 | 1190 | 1544 | 1 | 357 | |
| 5.14e-77 | 1079 | 1544 | 104 | 570 | |
| 2.16e-76 | 1079 | 1407 | 70 | 427 | |
| 6.33e-76 | 1081 | 1405 | 71 | 452 | |
| 8.02e-75 | 1079 | 1398 | 96 | 459 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.92e-39 | 397 | 856 | 18 | 403 | Chain A, Subtilase SubHal from Bacillus halmapalus [Sutcliffiella halmapala],5FAX_B Chain B, Subtilase SubHal from Bacillus halmapalus [Sutcliffiella halmapala],5FBZ_A Chain A, Enzyme subtilase SubHal from Bacillus halmapalus [Sutcliffiella halmapala],5FBZ_C Chain C, Enzyme subtilase SubHal from Bacillus halmapalus [Sutcliffiella halmapala] |
|
| 8.19e-37 | 381 | 856 | 1 | 404 | Crystal Structure of alkaline serine protease KP-43 from Bacillus sp. KSM-KP43 (1.30 angstrom, 100 K) [Bacillus sp. KSM-KP43],1WME_A Crystal Structure of alkaline serine protease KP-43 from Bacillus sp. KSM-KP43 (1.50 angstrom, 293 K) [Bacillus sp. KSM-KP43] |
|
| 2.71e-36 | 381 | 856 | 1 | 404 | Crystal Structure of alkaline serine protease KP-43 from Bacillus sp. KSM-KP43 (oxidized form, 1.73 angstrom) [Bacillus sp. KSM-KP43] |
|
| 3.15e-14 | 380 | 688 | 119 | 387 | Chain A, Subtilisin-like serine protease [Thermococcus kodakarensis],3AFG_B Chain B, Subtilisin-like serine protease [Thermococcus kodakarensis] |
|
| 1.52e-11 | 383 | 672 | 6 | 233 | Subtilisin-Carlsberg Complexed With Xenon (8 Bar) [Bacillus licheniformis],3UNX_A Bond length analysis of asp, glu and his residues in subtilisin Carlsberg at 1.26A resolution [Bacillus licheniformis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.12e-33 | 398 | 855 | 327 | 869 | Serine protease/ABC transporter B family protein tagD OS=Dictyostelium discoideum OX=44689 GN=tagD PE=3 SV=1 |
|
| 6.56e-33 | 375 | 856 | 264 | 802 | Serine protease/ABC transporter B family protein tagA OS=Dictyostelium discoideum OX=44689 GN=tagA PE=1 SV=2 |
|
| 2.01e-30 | 398 | 856 | 376 | 913 | Serine protease/ABC transporter B family protein tagB OS=Dictyostelium discoideum OX=44689 GN=tagB PE=3 SV=2 |
|
| 1.91e-28 | 398 | 855 | 314 | 863 | Serine protease/ABC transporter B family protein tagC OS=Dictyostelium discoideum OX=44689 GN=tagC PE=2 SV=2 |
|
| 1.35e-14 | 1151 | 1363 | 208 | 469 | Alpha-1,2-mannosyltransferase MNN21 OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=MNN21 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.004918 | 0.995061 | CS pos: 36-37. Pr: 0.9044 |