You are browsing environment: FUNGIDB
CAZyme Information: EPrPIT00000023155-p1
You are here: Home > Sequence: EPrPIT00000023155-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Globisporangium irregulare | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Oomycota; NA; ; Pythiaceae; Globisporangium; Globisporangium irregulare | |||||||||||
| CAZyme ID | EPrPIT00000023155-p1 | |||||||||||
| CAZy Family | GH81 | |||||||||||
| CAZyme Description | Catalase-peroxidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 459 | 681 | 9e-30 | 0.8862745098039215 |
| AA2 | 77 | 188 | 3.2e-24 | 0.42745098039215684 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 223453 | KatG | 0.0 | 54 | 684 | 66 | 728 | Catalase (peroxidase I) [Inorganic ion transport and metabolism]. |
| 237891 | PRK15061 | 0.0 | 54 | 681 | 53 | 721 | catalase/peroxidase. |
| 272957 | cat_per_HPI | 7.14e-173 | 55 | 684 | 52 | 712 | catalase/peroxidase HPI. As catalase, this enzyme catalyzes the dismutation of two molecules of hydrogen peroxide to dioxygen and two molecules of water. As a peroxidase, it uses hydrogen peroxide to oxidize donor compounds and produce water. KatG from E. coli is a homotetramer with two non-covalently associated iron protoheme IX groups per tetramer, but the ortholog from Synechococcus sp. is a homodimer with one protoheme. Important sites (numbered according to E. coli KatG) include heme ligands His-106 and His-267 and active site Trp-318. Note that the translation PID:g296476 from accession X71420 from Rhodobacter capsulatus B10 contains extensive frameshift differences from the rest of the orthologous family. [Cellular processes, Detoxification] |
| 173824 | catalase_peroxidase_1 | 2.44e-152 | 54 | 404 | 41 | 405 | N-terminal catalytic domain of catalase-peroxidases. This is a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Catalase-peroxidases can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. These enzymes are found in many archaeal and bacterial organisms, where they neutralize potentially lethal hydrogen peroxide molecules generated during photosynthesis or stationary phase. Along with related intracellular fungal and plant peroxidases, catalase-peroxidases belong to class I of the plant peroxidase superfamily. Unlike the eukaryotic enzymes, they are typically comprised of two homologous domains that probably arose via a single gene duplication event. The heme binding motif is present only in the N-terminal domain; the function of the C-terminal domain is not clear. |
| 173823 | plant_peroxidase_like | 2.18e-56 | 77 | 389 | 12 | 255 | Heme-dependent peroxidases similar to plant peroxidases. Along with animal peroxidases, these enzymes belong to a group of peroxidases containing a heme prosthetic group (ferriprotoporphyrin IX), which catalyzes a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. The plant peroxidase-like superfamily is found in all three kingdoms of life and carries out a variety of biosynthetic and degradative functions. Several sub-families can be identified. Class I includes intracellular peroxidases present in fungi, plants, archaea and bacteria, called catalase-peroxidases, that can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. Catalase-peroxidases are typically comprised of two homologous domains that probably arose via a single gene duplication event. Class II includes ligninase and other extracellular fungal peroxidases, while class III is comprised of classic extracellular plant peroxidases, like horseradish peroxidase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 688 | 1 | 687 | |
| 0.0 | 1 | 688 | 1 | 687 | |
| 0.0 | 1 | 688 | 1 | 687 | |
| 1.05e-134 | 55 | 686 | 41 | 715 | |
| 7.67e-134 | 54 | 684 | 56 | 749 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.22e-135 | 55 | 686 | 59 | 731 | Crystal structure of catalase-peroxidase from Haloarcula marismortui [Haloarcula marismortui],1ITK_B Crystal structure of catalase-peroxidase from Haloarcula marismortui [Haloarcula marismortui] |
|
| 8.70e-135 | 54 | 684 | 68 | 755 | Crystal structure of the catalase-peroxidase from Neurospora crassa at 2.9 A [Neurospora crassa OR74A],5WHQ_B Crystal structure of the catalase-peroxidase from Neurospora crassa at 2.9 A [Neurospora crassa OR74A] |
|
| 2.05e-134 | 55 | 686 | 59 | 731 | Crystal Structure Analysis of the Ser305Thr Variants of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049],3UW8_B Crystal Structure Analysis of the Ser305Thr Variants of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049] |
|
| 2.05e-134 | 55 | 686 | 59 | 731 | Crystal Structure Analysis of the Ser305Ala variant of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049],3VLK_B Crystal Structure Analysis of the Ser305Ala variant of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049],3VLL_A Crystal Structure Analysis of the Ser305Ala variant of KatG from HALOARCULA MARISMORTUI Complexes with Inhibitor SHA [Haloarcula marismortui ATCC 43049],3VLL_B Crystal Structure Analysis of the Ser305Ala variant of KatG from HALOARCULA MARISMORTUI Complexes with Inhibitor SHA [Haloarcula marismortui ATCC 43049] |
|
| 5.73e-134 | 55 | 686 | 59 | 731 | Crystal Structure Analysis of the Met244Ala Variant of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049],3VLM_B Crystal Structure Analysis of the Met244Ala Variant of KatG from Haloarcula marismortui [Haloarcula marismortui ATCC 43049] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.81e-147 | 54 | 684 | 53 | 749 | Catalase-peroxidase OS=Pseudomonas putida (strain ATCC 47054 / DSM 6125 / CFBP 8728 / NCIMB 11950 / KT2440) OX=160488 GN=katG PE=3 SV=1 |
|
| 8.81e-147 | 54 | 684 | 53 | 749 | Catalase-peroxidase OS=Pseudomonas putida (strain ATCC 700007 / DSM 6899 / BCRC 17059 / F1) OX=351746 GN=katG PE=3 SV=1 |
|
| 2.85e-146 | 54 | 684 | 54 | 742 | Catalase-peroxidase OS=Pseudomonas entomophila (strain L48) OX=384676 GN=katG PE=3 SV=1 |
|
| 3.64e-145 | 54 | 684 | 70 | 739 | Catalase-peroxidase 2 OS=Legionella pneumophila OX=446 GN=katG2 PE=2 SV=1 |
|
| 3.64e-145 | 54 | 684 | 70 | 739 | Catalase-peroxidase 2 OS=Legionella pneumophila subsp. pneumophila (strain Philadelphia 1 / ATCC 33152 / DSM 7513) OX=272624 GN=katG2 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
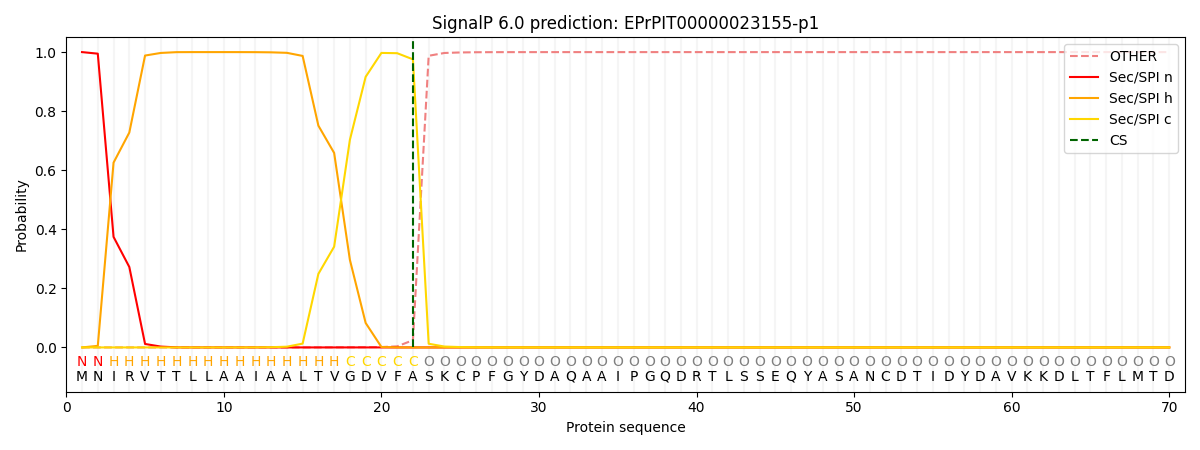
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000218 | 0.999770 | CS pos: 22-23. Pr: 0.9755 |
