You are browsing environment: FUNGIDB
CAZyme Information: ELR08916.1
You are here: Home > Sequence: ELR08916.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
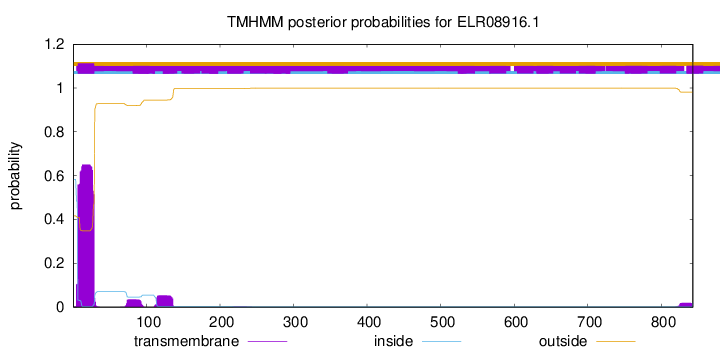
TMHMM annotations
Basic Information help
| Species | Pseudogymnoascus destructans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Leotiomycetes; ; Pseudeurotiaceae; Pseudogymnoascus; Pseudogymnoascus destructans | |||||||||||
| CAZyme ID | ELR08916.1 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | GH16 domain-containing protein [Source:UniProtKB/TrEMBL;Acc:L8GA54] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.73:4 | 3.2.1.6:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH16 | 73 | 321 | 7.9e-73 | 0.982532751091703 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 185690 | GH16_fungal_Lam16A_glucanase | 4.60e-125 | 32 | 342 | 1 | 293 | fungal 1,3(4)-beta-D-glucanases, similar to Phanerochaete chrysosporium laminarinase 16A. Group of fungal 1,3(4)-beta-D-glucanases, similar to Phanerochaete chrysosporium laminarinase 16A. Lam16A belongs to the 'nonspecific' 1,3(4)-beta-glucanase subfamily, although beta-1,6 branching and beta-1,4 bonds specifically define where Lam16A hydrolyzes its substrates, like curdlan (beta-1,3-glucan), lichenin (beta-1,3-1,4-mixed linkage glucan), and laminarin (beta-1,6-branched-1,3-glucan). |
| 185693 | GH16_laminarinase_like | 3.95e-11 | 129 | 299 | 92 | 218 | Laminarinase, member of the glycosyl hydrolase family 16. Laminarinase, also known as glucan endo-1,3-beta-D-glucosidase, is a glycosyl hydrolase family 16 member that hydrolyzes 1,3-beta-D-glucosidic linkages in 1,3-beta-D-glucans such as laminarins, curdlans, paramylons, and pachymans, with very limited action on mixed-link (1,3-1,4-)-beta-D-glucans. |
| 185691 | GH16_Strep_laminarinase_like | 6.68e-09 | 130 | 169 | 106 | 151 | Streptomyces laminarinase-like, member of glycosyl hydrolase family 16. Proteins similar to Streptomyces sioyaensis beta-1,3-glucanase (laminarinase) present in Actinomycetales as well as Peziomycotina. Laminarinases belong to glycosyl hydrolase family 16 and hydrolyze the glycosidic bond of the 1,3-beta-linked glucan, a major component of fungal and plant cell walls and the structural and storage polysaccharides (laminarin) of marine macro-algae. Members of the GH16 family have a conserved jelly roll fold with an active site channel. |
| 397207 | Amelogenin | 4.64e-06 | 747 | 819 | 84 | 153 | Amelogenin. Amelogenins play a role in biomineralisation. They seem to regulate the formation of crystallites during the secretory stage of tooth enamel development. thought to play a major role in the structural organisation and mineralisation of developing enamel. They are found in the extracellular matrix. Mutations in X-chromosomal amelogenin can cause Amelogenesis imperfecta. |
| 185694 | GH16_CCF | 0.001 | 132 | 239 | 130 | 222 | Coelomic cytolytic factor, member of glycosyl hydrolase family 16. Subgroup of glucanases of unknown function that are related to beta-GRP (beta-1,3-glucan recognition protein), but contain active site residues. Beta-GRPs are one group of pattern recognition receptors (PRRs), also referred to as biosensor proteins, that complexes with pathogen-associated beta-1,3-glucans and then transduces signals necessary for activation of an appropriate innate immune response. Beta-GRPs are present in insects and lack all catalytic residues. This subgroup contains related proteins that still contain the active site and are widely distributed in eukaryotes. Their structures adopt a jelly roll fold with a deep active site channel harboring the catalytic residues, like those of other glycosyl hydrolase family 16 members. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.04e-91 | 37 | 342 | 28 | 336 | |
| 3.57e-78 | 33 | 344 | 22 | 317 | |
| 9.63e-78 | 43 | 342 | 33 | 311 | |
| 9.93e-78 | 43 | 342 | 33 | 311 | |
| 9.93e-78 | 43 | 342 | 33 | 311 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.19e-74 | 33 | 341 | 2 | 295 | The complex structure of PtLic16A with cellobiose [Paecilomyces sp. 'thermophila'],3WDU_B The complex structure of PtLic16A with cellobiose [Paecilomyces sp. 'thermophila'],3WDU_C The complex structure of PtLic16A with cellobiose [Paecilomyces sp. 'thermophila'],3WDU_D The complex structure of PtLic16A with cellobiose [Paecilomyces sp. 'thermophila'] |
|
| 3.29e-74 | 33 | 341 | 3 | 296 | The apo-form structure of PtLic16A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDT_B The apo-form structure of PtLic16A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDT_C The apo-form structure of PtLic16A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDT_D The apo-form structure of PtLic16A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDV_A The complex structure of PtLic16A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDV_B The complex structure of PtLic16A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDV_C The complex structure of PtLic16A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDV_D The complex structure of PtLic16A with cellotetraose [Paecilomyces sp. 'thermophila'] |
|
| 2.25e-73 | 33 | 341 | 1 | 294 | The complex structure of E113A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDY_B The complex structure of E113A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDY_C The complex structure of E113A with cellotetraose [Paecilomyces sp. 'thermophila'],3WDY_D The complex structure of E113A with cellotetraose [Paecilomyces sp. 'thermophila'] |
|
| 2.39e-73 | 33 | 341 | 3 | 296 | The apo-form structure of E113A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDW_B The apo-form structure of E113A from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],3WDX_A The complex structure of E113A with glucotriose [Paecilomyces sp. 'thermophila'],3WDX_B The complex structure of E113A with glucotriose [Paecilomyces sp. 'thermophila'] |
|
| 4.70e-70 | 33 | 341 | 3 | 297 | Crystal structure and characterization an elongating GH family 16 beta-1,3-glucosyltransferase [Paecilomyces sp. 'thermophila'],5JVV_B Crystal structure and characterization an elongating GH family 16 beta-1,3-glucosyltransferase [Paecilomyces sp. 'thermophila'] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.01e-73 | 1 | 343 | 1 | 328 | Probable endo-1,3(4)-beta-glucanase AFUB_029980 OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=AFUB_029980 PE=3 SV=1 |
|
| 1.01e-73 | 1 | 343 | 1 | 328 | Probable endo-1,3(4)-beta-glucanase AFUA_2G14360 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=AFUA_2G14360 PE=3 SV=1 |
|
| 2.53e-72 | 1 | 343 | 1 | 328 | Probable endo-1,3(4)-beta-glucanase NFIA_089530 OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=NFIA_089530 PE=3 SV=1 |
|
| 3.26e-70 | 33 | 342 | 20 | 315 | Endo-1,3(4)-beta-glucanase ARB_04519 OS=Arthroderma benhamiae (strain ATCC MYA-4681 / CBS 112371) OX=663331 GN=ARB_04519 PE=3 SV=1 |
|
| 4.32e-68 | 1 | 342 | 1 | 328 | Probable endo-1,3(4)-beta-glucanase ACLA_073210 OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=ACLA_073210 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
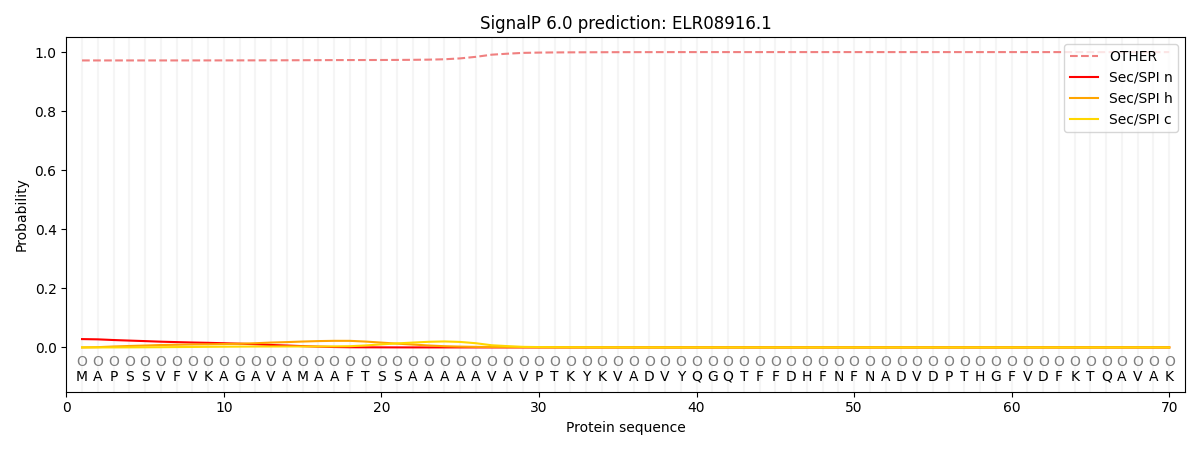
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.973964 | 0.026070 |