You are browsing environment: FUNGIDB
CAZyme Information: EKG20856.1
You are here: Home > Sequence: EKG20856.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
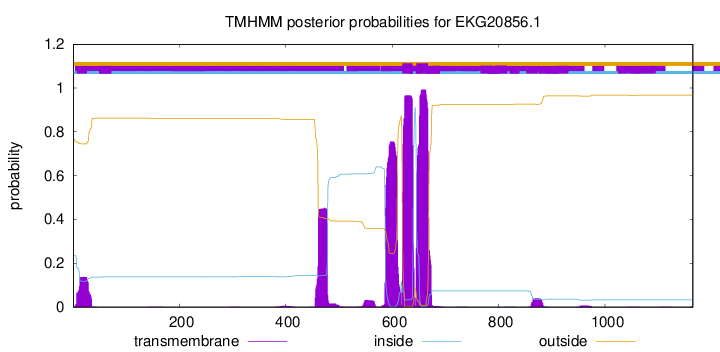
TMHMM annotations
Basic Information help
| Species | Macrophomina phaseolina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Botryosphaeriaceae; Macrophomina; Macrophomina phaseolina | |||||||||||
| CAZyme ID | EKG20856.1 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | Cytochrome P450 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.55:10 |
|---|
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 410684 | CYP67-like | 2.12e-132 | 715 | 1156 | 1 | 417 | cytochrome P450 family 67 and similar cytochrome P450s. This subfamily includes Uromyces viciae-fabae cytochrome P450 67 (CYP67), also called planta-induced rust protein 16, Cystobasidium minutum (Rhodotorula minuta) cytochrome P450rm, and other fungal cytochrome P450s. P450rm catalyzes the formation of isobutene and 4-hydroxylation of benzoate. The gene encoding CYP67 is a planta-induced gene that is expressed in haustoria and rust-infected leaves. The CYP67-like subfamily belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410683 | CYP57A1-like | 8.82e-89 | 715 | 1145 | 1 | 417 | cytochrome P450 family 57, subfamily A, polypeptide 1 and similar cytochrome P450s. This family is composed of fungal cytochrome P450s including: Nectria haematococca cytochrome P450 57A1 (CYP57A1), also called pisatin demethylase, which detoxifies the phytoalexin pisatin; Penicillium aethiopicum P450 monooxygenase gsfF, also called griseofulvin synthesis protein F, which catalyzes the coupling of orcinol and phloroglucinol rings in griseophenone B to form desmethyl-dehydrogriseofulvin A during the biosynthesis of griseofulvin, a spirocyclic fungal natural product used to treat dermatophyte infections; and Penicillium aethiopicum P450 monooxygenase vrtE, also called viridicatumtoxin synthesis protein E, which catalyzes hydroxylation at C5 of the polyketide backbone during the biosynthesis of viridicatumtoxin, a tetracycline-like fungal meroterpenoid. The CYP57A1-like family belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410681 | CYP60B-like | 7.75e-76 | 715 | 1132 | 1 | 396 | cytochrome P450 family 60, subfamily B and similar cytochrome P450s. This family is composed of fungal cytochrome P450s including: Aspergillus nidulans cytochrome P450 60B (CYP60B), also called versicolorin B desaturase, which catalyzes the conversion of versicolorin B to versicolorin A during sterigmatocystin biosynthesis; Fusarium sporotrichioides cytochrome P450 65A1 (CYP65A1), also called isotrichodermin C-15 hydroxylase, which catalyzes the hydroxylation at C-15 of isotricodermin in trichothecene biosynthesis; and Penicillium aethiopicum P450 monooxygenase vrtK, also called viridicatumtoxin synthesis protein K, which catalyzes the spirocyclization of the geranyl moiety of previridicatumtoxin to produce viridicatumtoxin, a tetracycline-like fungal meroterpenoid. The CYP60B-like family belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410685 | CYP58-like | 4.14e-62 | 718 | 1141 | 4 | 410 | cytochrome P450 family 58-like fungal cytochrome P450s. This group includes Fusarium sporotrichioides cytochrome P450 58 (CYP58, also known as Tri4 and trichodiene oxygenase), and similar fungal proteins. CYP58 catalyzes the oxygenation of trichodiene during the biosynthesis of trichothecenes, which are sesquiterpenoid toxins that act by inhibiting protein biosynthesis. The CYP58-like subfamily belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410682 | CYP_fungal | 1.65e-58 | 715 | 1135 | 1 | 403 | unknown subfamily of fungal cytochrome P450s. This subfamily is composed of uncharacterized fungal cytochrome P450s. Cytochrome P450 (P450, CYP) is a large superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. Their monooxygenase activity relies on the reductive scission of molecular oxygen bound to the P450 heme iron, and the delivery of two electrons to the heme iron during the catalytic cycle. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.93e-245 | 21 | 574 | 23 | 570 | |
| 3.93e-245 | 21 | 574 | 23 | 570 | |
| 3.93e-245 | 21 | 574 | 23 | 570 | |
| 1.41e-242 | 21 | 574 | 23 | 570 | |
| 2.55e-242 | 21 | 574 | 23 | 577 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.53e-125 | 24 | 574 | 1 | 563 | Chain AAA, MgGH51 [Meripilus giganteus],6ZPV_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPW_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPX_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPY_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPZ_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ0_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ1_AAA Chain AAA, MgGH51 [Meripilus giganteus] |
|
| 4.42e-11 | 760 | 1127 | 94 | 441 | Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_B Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_C Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_D Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_E Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_F Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_G Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_H Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_I Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_J Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_K Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],5VEU_L Human Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],6MJM_A Substrate Free Cytochrome P450 3A5 (CYP3A5) [Homo sapiens],7SV2_A Chain A, Cytochrome P450 3A5 [Homo sapiens],7SV2_B Chain B, Cytochrome P450 3A5 [Homo sapiens],7SV2_C Chain C, Cytochrome P450 3A5 [Homo sapiens],7SV2_D Chain D, Cytochrome P450 3A5 [Homo sapiens] |
|
| 4.44e-11 | 760 | 1127 | 95 | 442 | Chain A, Cytochrome P450 3A5 [Homo sapiens] |
|
| 1.56e-09 | 967 | 1130 | 278 | 429 | Chain A, Bifunctional P-450/NADPH-P450 reductase [Priestia megaterium],4RSN_B Chain B, Bifunctional P-450/NADPH-P450 reductase [Priestia megaterium] |
|
| 2.79e-09 | 966 | 1164 | 286 | 471 | Crystal structure of a Cytochrome P450 (CYP102L1) [Mycobacterium phage Adler],6N6Q_B Crystal structure of a Cytochrome P450 (CYP102L1) [Mycobacterium phage Adler],6N6Q_C Crystal structure of a Cytochrome P450 (CYP102L1) [Mycobacterium phage Adler],6N6Q_D Crystal structure of a Cytochrome P450 (CYP102L1) [Mycobacterium phage Adler] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.98e-246 | 21 | 574 | 23 | 570 | Probable alpha-L-arabinofuranosidase A OS=Aspergillus flavus (strain ATCC 200026 / FGSC A1120 / IAM 13836 / NRRL 3357 / JCM 12722 / SRRC 167) OX=332952 GN=abfA PE=3 SV=2 |
|
| 1.57e-244 | 21 | 574 | 23 | 570 | Probable alpha-L-arabinofuranosidase A OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=abfA PE=3 SV=2 |
|
| 8.56e-244 | 21 | 574 | 23 | 569 | Probable alpha-L-arabinofuranosidase A OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=abfA PE=3 SV=1 |
|
| 4.34e-241 | 24 | 574 | 26 | 569 | Alpha-L-arabinofuranosidase A OS=Aspergillus niger OX=5061 GN=abfA PE=1 SV=1 |
|
| 8.66e-241 | 24 | 574 | 26 | 569 | Probable alpha-L-arabinofuranosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=abfA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000232 | 0.999737 | CS pos: 23-24. Pr: 0.9569 |