You are browsing environment: FUNGIDB
CAZyme Information: EKG10244.1
You are here: Home > Sequence: EKG10244.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Macrophomina phaseolina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Dothideomycetes; ; Botryosphaeriaceae; Macrophomina; Macrophomina phaseolina | |||||||||||
| CAZyme ID | EKG10244.1 | |||||||||||
| CAZy Family | AA11 | |||||||||||
| CAZyme Description | Glycoside hydrolase family 16 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH16 | 180 | 304 | 1.2e-51 | 0.6614583333333334 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 185683 | Glyco_hydrolase_16 | 4.35e-38 | 109 | 304 | 39 | 210 | glycosyl hydrolase family 16. The O-Glycosyl hydrolases are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A glycosyl hydrolase classification system based on sequence similarity has led to the definition of more than 95 different families inlcuding glycosyl hydrolase family 16. Family 16 includes lichenase, xyloglucan endotransglycosylase (XET), beta-agarase, kappa-carrageenase, endo-beta-1,3-glucanase, endo-beta-1,3-1,4-glucanase, and endo-beta-galactosidase, all of which have a conserved jelly roll fold with a deep active site channel harboring the catalytic residues. |
| 185684 | GH16_lichenase | 2.60e-12 | 98 | 300 | 29 | 205 | lichenase, member of glycosyl hydrolase family 16. Lichenase, also known as 1,3-1,4-beta-glucanase, is a member of glycosyl hydrolase family 16, that specifically cleaves 1,4-beta-D-glucosidic bonds in mixed-linked beta glucans that also contain 1,3-beta-D-glucosidic linkages. Natural substrates of beta-glucanase are beta-glucans from grain endosperm cell walls or lichenan from the Islandic moss, Cetraria islandica. This protein is found not only in bacteria but also in anaerobic fungi. This domain includes two seven-stranded antiparallel beta-sheets that are adjacent to one another forming a compact, jellyroll beta-sandwich structure. |
| 225182 | BglS | 7.86e-07 | 126 | 376 | 106 | 327 | Beta-glucanase, GH16 family [Carbohydrate transport and metabolism]. |
| 185693 | GH16_laminarinase_like | 9.04e-07 | 128 | 304 | 69 | 235 | Laminarinase, member of the glycosyl hydrolase family 16. Laminarinase, also known as glucan endo-1,3-beta-D-glucosidase, is a glycosyl hydrolase family 16 member that hydrolyzes 1,3-beta-D-glucosidic linkages in 1,3-beta-D-glucans such as laminarins, curdlans, paramylons, and pachymans, with very limited action on mixed-link (1,3-1,4-)-beta-D-glucans. |
| 395585 | Glyco_hydro_16 | 2.23e-06 | 131 | 257 | 26 | 123 | Glycosyl hydrolases family 16. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.37e-111 | 9 | 371 | 9 | 350 | |
| 5.32e-110 | 9 | 372 | 8 | 348 | |
| 1.61e-109 | 21 | 370 | 20 | 347 | |
| 1.56e-94 | 4 | 381 | 2 | 361 | |
| 2.21e-94 | 23 | 382 | 21 | 363 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.42e-06 | 111 | 304 | 55 | 234 | Crystal structure of laminarinase from Flavobacterium sp. UMI-01 [Flavobacterium sp.],5WUT_B Crystal structure of laminarinase from Flavobacterium sp. UMI-01 [Flavobacterium sp.] |
|
| 9.26e-06 | 227 | 285 | 133 | 192 | Bacillus Licheniformis Beta-Glucanase [Bacillus licheniformis] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
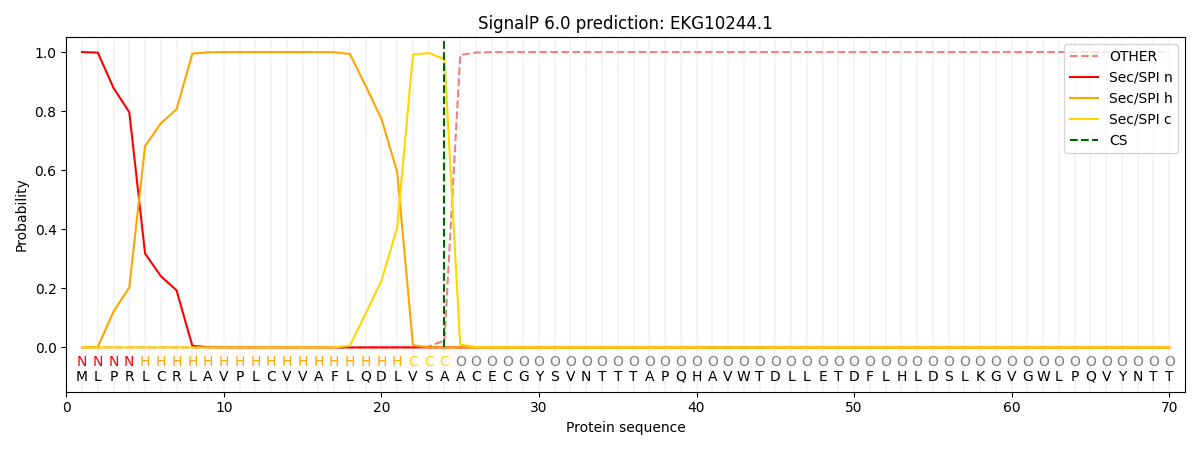
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000211 | 0.999779 | CS pos: 24-25. Pr: 0.9762 |
