You are browsing environment: FUNGIDB
CAZyme Information: EJT81576.1
You are here: Home > Sequence: EJT81576.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Gaeumannomyces tritici | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Magnaporthaceae; Gaeumannomyces; Gaeumannomyces tritici | |||||||||||
| CAZyme ID | EJT81576.1 | |||||||||||
| CAZy Family | GT20 | |||||||||||
| CAZyme Description | Phosphomevalonate kinase [Source:UniProtKB/TrEMBL;Acc:J3NJX2] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 306 | 513 | 1.8e-42 | 0.4126637554585153 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 235028 | PRK02304 | 1.75e-34 | 964 | 1155 | 1 | 175 | adenine phosphoribosyltransferase; Provisional |
| 223577 | Apt | 8.67e-28 | 966 | 1155 | 5 | 179 | Adenine/guanine phosphoribosyltransferase or related PRPP-binding protein [Nucleotide transport and metabolism]. |
| 177930 | PLN02293 | 1.33e-21 | 966 | 1134 | 14 | 170 | adenine phosphoribosyltransferase |
| 398111 | P-mevalo_kinase | 2.30e-19 | 775 | 893 | 1 | 111 | Phosphomevalonate kinase. Phosphomevalonate kinase (EC:2.7.4.2) catalyzes the phosphorylation of 5-phosphomevalonate into 5-diphosphomevalonate, an essential step in isoprenoid biosynthesis via the mevalonate pathway. This family represents the animal type of the enzyme. The other is the ERG8 type, found in plants and fungi, and some bacteria (see pfam00288). |
| 206754 | PRTases_typeI | 6.59e-19 | 1002 | 1136 | 1 | 118 | Phosphoribosyl transferase (PRT)-type I domain. Phosphoribosyl transferase (PRT) domain. The type I PRTases are identified by a conserved PRPP binding motif which features two adjacent acidic residues surrounded by one or more hydrophobic residue. PRTases catalyze the displacement of the alpha-1'-pyrophosphate of 5-phosphoribosyl-alpha1-pyrophosphate (PRPP) by a nitrogen-containing nucleophile. The reaction products are an alpha-1 substituted ribose-5'-phosphate and a free pyrophosphate (PP). PRPP, an activated form of ribose-5-phosphate, is a key metabolite connecting nucleotide synthesis and salvage pathways. The type I PRTase family includes a range of diverse phosphoribosyl transferase enzymes and regulatory proteins of the nucleotide synthesis and salvage pathways, including adenine phosphoribosyltransferase EC:2.4.2.7., hypoxanthine-guanine-xanthine phosphoribosyltransferase, hypoxanthine phosphoribosyltransferase EC:2.4.2.8., ribose-phosphate pyrophosphokinase EC:2.7.6.1., amidophosphoribosyltransferase EC:2.4.2.14., orotate phosphoribosyltransferase EC:2.4.2.10., uracil phosphoribosyltransferase EC:2.4.2.9., and xanthine-guanine phosphoribosyltransferase EC:2.4.2.22. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.00e-16 | 314 | 503 | 119 | 290 | |
| 1.86e-14 | 316 | 503 | 62 | 233 | |
| 1.86e-14 | 316 | 503 | 62 | 233 | |
| 1.86e-14 | 316 | 503 | 62 | 233 | |
| 1.86e-14 | 316 | 503 | 62 | 233 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.51e-18 | 965 | 1137 | 3 | 166 | Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 14 hours post crystallization [Homo sapiens],6FD4_B Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 14 hours post crystallization [Homo sapiens],6FD5_A Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 14 days post crystallization (with AMP and PPi products partially generated) [Homo sapiens],6FD5_B Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 14 days post crystallization (with AMP and PPi products partially generated) [Homo sapiens],6FD6_A Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 30 days post crystallization (with AMP and PPi products fully generated) [Homo sapiens],6FD6_B Crystal Structure of Human APRT-Tyr105Phe variant in complex with Adenine, PRPP and Mg2+, 30 days post crystallization (with AMP and PPi products fully generated) [Homo sapiens] |
|
| 1.51e-18 | 965 | 1137 | 3 | 166 | Crystal Structure of Human APRT wild type in complex with PRPP and Mg2+ [Homo sapiens],6FCH_B Crystal Structure of Human APRT wild type in complex with PRPP and Mg2+ [Homo sapiens],6FCL_A Crystal Structure of Human APRT wild type in complex with AMP [Homo sapiens],6FCL_B Crystal Structure of Human APRT wild type in complex with AMP [Homo sapiens],6HGP_A Crystal Structure of Human APRT wild type in complex with Phosphate ion. [Homo sapiens],6HGP_B Crystal Structure of Human APRT wild type in complex with Phosphate ion. [Homo sapiens],6HGR_A Crystal Structure of Human APRT wild type in complex with IMP [Homo sapiens],6HGR_B Crystal Structure of Human APRT wild type in complex with IMP [Homo sapiens],6HGS_A Crystal Structure of Human APRT wild type in complex with GMP [Homo sapiens],6HGS_B Crystal Structure of Human APRT wild type in complex with GMP [Homo sapiens] |
|
| 1.55e-18 | 965 | 1137 | 4 | 167 | Crystal Structure of Human APRT wild type in complex with Adenine, PRPP and Mg2+ [Homo sapiens],6FCI_B Crystal Structure of Human APRT wild type in complex with Adenine, PRPP and Mg2+ [Homo sapiens],6FCI_C Crystal Structure of Human APRT wild type in complex with Adenine, PRPP and Mg2+ [Homo sapiens],6FCI_D Crystal Structure of Human APRT wild type in complex with Adenine, PRPP and Mg2+ [Homo sapiens],6HGQ_A Crystal Structure of Human APRT wild type in complex with Hypoxanthine, PRPP and Mg2+ [Homo sapiens],6HGQ_B Crystal Structure of Human APRT wild type in complex with Hypoxanthine, PRPP and Mg2+ [Homo sapiens],6HGQ_C Crystal Structure of Human APRT wild type in complex with Hypoxanthine, PRPP and Mg2+ [Homo sapiens],6HGQ_D Crystal Structure of Human APRT wild type in complex with Hypoxanthine, PRPP and Mg2+ [Homo sapiens] |
|
| 1.59e-18 | 965 | 1137 | 5 | 168 | Crystal Structure of F173G Mutant of Human APRT [Homo sapiens],4X45_B Crystal Structure of F173G Mutant of Human APRT [Homo sapiens] |
|
| 1.59e-18 | 965 | 1137 | 5 | 168 | Human Adenine Phosphoribosyltransferase [Homo sapiens],1ZN7_A Human Adenine Phosphoribosyltransferase Complexed with PRPP, ADE and R5P [Homo sapiens],1ZN7_B Human Adenine Phosphoribosyltransferase Complexed with PRPP, ADE and R5P [Homo sapiens],1ZN8_A Human Adenine Phosphoribosyltransferase Complexed with AMP, in Space Group P1 at 1.76 A Resolution [Homo sapiens],1ZN8_B Human Adenine Phosphoribosyltransferase Complexed with AMP, in Space Group P1 at 1.76 A Resolution [Homo sapiens],1ZN9_A Human Adenine Phosphoribosyltransferase in Apo and AMP Complexed Forms [Homo sapiens],1ZN9_B Human Adenine Phosphoribosyltransferase in Apo and AMP Complexed Forms [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6.69e-23 | 965 | 1153 | 5 | 179 | Adenine phosphoribosyltransferase OS=Cricetulus griseus OX=10029 GN=APRT PE=3 SV=2 |
|
| 9.11e-23 | 965 | 1153 | 5 | 179 | Adenine phosphoribosyltransferase OS=Mastomys natalensis OX=10112 GN=APRT PE=3 SV=1 |
|
| 1.69e-22 | 965 | 1153 | 5 | 179 | Adenine phosphoribosyltransferase OS=Dipodillus campestris OX=41199 GN=APRT PE=3 SV=1 |
|
| 3.13e-22 | 965 | 1153 | 5 | 179 | Adenine phosphoribosyltransferase OS=Rattus norvegicus OX=10116 GN=Aprt PE=1 SV=1 |
|
| 4.26e-22 | 965 | 1153 | 5 | 179 | Adenine phosphoribosyltransferase OS=Bos taurus OX=9913 GN=APRT PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
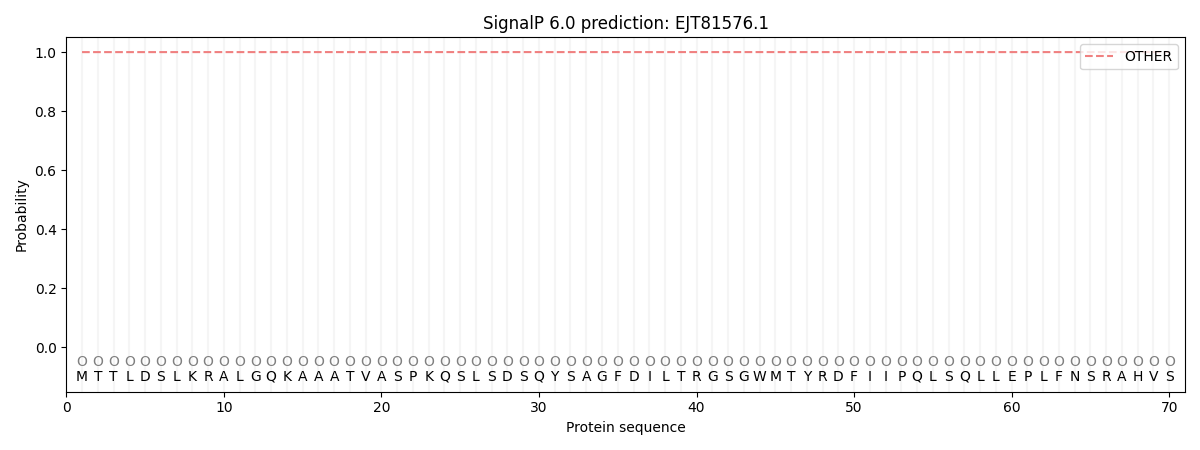
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000029 | 0.000000 |
