You are browsing environment: FUNGIDB
CAZyme Information: EAQ93007.1
You are here: Home > Sequence: EAQ93007.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
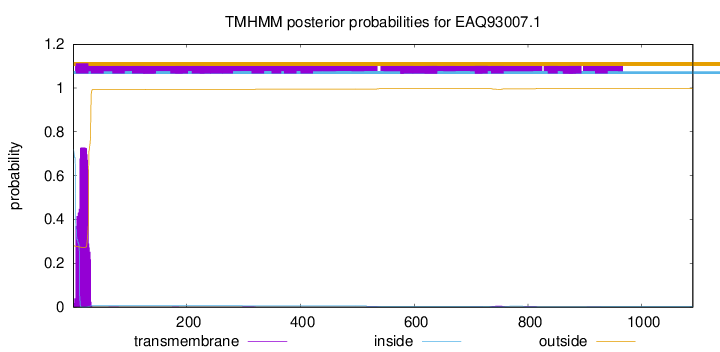
TMHMM annotations
Basic Information help
| Species | Chaetomium globosum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Chaetomiaceae; Chaetomium; Chaetomium globosum | |||||||||||
| CAZyme ID | EAQ93007.1 | |||||||||||
| CAZy Family | GT32 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 645 | 1065 | 4.5e-43 | 0.9497816593886463 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 410667 | CYP503A1-like | 6.13e-112 | 67 | 446 | 1 | 377 | cytochrome P450 family 503, subfamily A, polypeptide 1 and similar cytochrome P450s. This family is composed of predominantly fungal cytochrome P450s (CYPs) with similarity to Fusarium fujikuroi Cytochrome P450 503A1 (CYP503A1, also called ent-kaurene oxidase or cytochrome P450-4), Aspergillus nidulans austinol synthesis protein I (ausI), Alternaria alternata tentoxin synthesis protein 1 (TES1), and Acanthamoeba polyphaga mimivirus cytochrome P450 51 (CYP51, also called P450-LIA1 or sterol 14-alpha demethylase). Ent-kaurene oxidase catalyzes three successive oxidations of the 4-methyl group of ent-kaurene to form kaurenoic acid, an intermediate in gibberellin biosynthesis. AusI and TES1 are cytochrome P450 monooxygenases that mediate the biosynthesis of the meroterpenoids, austinol and dehydroaustinol, and the phytotoxin tentoxin, respectively. P450-LIA1 catalyzes the 14-alpha demethylation of obtusifoliol and functions in steroid biosynthesis. This family belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
| 410651 | cytochrome_P450 | 3.38e-25 | 145 | 442 | 73 | 337 | cytochrome P450 (CYP) superfamily. Cytochrome P450 (P450, CYP) is a large superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs with > 40% sequence identity are members of the same family. There are approximately 2250 CYP families: mammals, insects, plants, fungi, bacteria, and archaea have around 18, 208, 277, 805, 591, and 14 families, respectively. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. Their monooxygenase activity relies on the reductive scission of molecular oxygen bound to the P450 heme iron, and the delivery of two electrons to the heme iron during the catalytic cycle. CYPs use a variety of redox partners, such as the eukaryotic diflavin enzyme NADPH-cytochrome P450 oxidoreductase and the bacterial/mitochondrial NAD(P)H-ferredoxin reductase and ferredoxin partners. Some CYPs are naturally linked to their redox partners and others have evolved to bypass requirements for redox partners, and instead react directly with hydrogen peroxide or NAD(P)H to facilitate oxidative or reductive catalysis. |
| 395020 | p450 | 8.81e-24 | 192 | 456 | 164 | 412 | Cytochrome P450. Cytochrome P450s are haem-thiolate proteins involved in the oxidative degradation of various compounds. They are particularly well known for their role in the degradation of environmental toxins and mutagens. They can be divided into 4 classes, according to the method by which electrons from NAD(P)H are delivered to the catalytic site. Sequence conservation is relatively low within the family - there are only 3 absolutely conserved residues - but their general topography and structural fold are highly conserved. The conserved core is composed of a coil termed the 'meander', a four-helix bundle, helices J and K, and two sets of beta-sheets. These constitute the haem-binding loop (with an absolutely conserved cysteine that serves as the 5th ligand for the haem iron), the proton-transfer groove and the absolutely conserved EXXR motif in helix K. While prokaryotic P450s are soluble proteins, most eukaryotic P450s are associated with microsomal membranes. their general enzymatic function is to catalyze regiospecific and stereospecific oxidation of non-activated hydrocarbons at physiological temperatures. |
| 223354 | GlcD | 1.33e-19 | 624 | 1062 | 24 | 451 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 410668 | CYP51-like | 1.93e-18 | 236 | 442 | 156 | 357 | cytochrome P450 family 51 and similar cytochrome P450s. This family is composed of cytochrome P450 51 (CYP51 or sterol 14alpha-demethylase) and related cytochrome P450s. CYP51 is the only cytochrome P450 enzyme with a conserved function across animals, fungi, and plants, in the synthesis of essential sterols. In mammals, it is expressed in many different tissues, with highest expression in testis, ovary, adrenal gland, prostate, liver, kidney, and lung. In fungi, CYP51 is a significant drug target for treatment of human protozoan infections. In plants, it functions within a specialized defense-related metabolic pathway. CYP51 is also found in several bacterial species. This family belongs to the large cytochrome P450 (P450, CYP) superfamily of heme-containing proteins that catalyze a variety of oxidative reactions of a large number of structurally different endogenous and exogenous compounds in organisms from all major domains of life. CYPs bind their diverse ligands in a buried, hydrophobic active site, which is accessed through a substrate access channel formed by two flexible helices and their connecting loop. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 2.85e-30 | 528 | 1073 | 500 | 1054 | |
| 2.85e-30 | 528 | 1073 | 500 | 1054 | |
| 5.04e-16 | 20 | 428 | 15 | 432 | |
| 2.41e-15 | 646 | 817 | 27 | 188 | |
| 1.40e-12 | 647 | 818 | 56 | 217 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.59e-55 | 528 | 1061 | 28 | 552 | Crystal structure of VAO-type flavoprotein MtVAO615 at pH 7.5 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],6F73_A Crystal structure of VAO-type flavoprotein MtVAO615 at pH 5.0 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],6F73_B Crystal structure of VAO-type flavoprotein MtVAO615 at pH 5.0 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464] |
|
| 1.76e-34 | 528 | 1071 | 30 | 586 | Crystal structure of VAO-type flavoprotein MtVAO713 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],6F74_B Crystal structure of VAO-type flavoprotein MtVAO713 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],6F74_C Crystal structure of VAO-type flavoprotein MtVAO713 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],6F74_D Crystal structure of VAO-type flavoprotein MtVAO713 from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464] |
|
| 3.68e-12 | 648 | 835 | 63 | 240 | Chain A, MaDA [Morus alba],6JQH_B Chain B, MaDA [Morus alba] |
|
| 5.47e-12 | 647 | 1062 | 53 | 454 | The crystal structure of EncM H138T mutant [Streptomyces maritimus],6FYE_B The crystal structure of EncM H138T mutant [Streptomyces maritimus] |
|
| 7.23e-12 | 647 | 1062 | 53 | 454 | The crystal structure of EncM L144M mutant [Streptomyces maritimus],6FYB_B The crystal structure of EncM L144M mutant [Streptomyces maritimus],6FYB_C The crystal structure of EncM L144M mutant [Streptomyces maritimus],6FYB_D The crystal structure of EncM L144M mutant [Streptomyces maritimus],6FYC_A The crystal structure of EncM L144M mutant complex with dioxygen under 15 bars O2 pressure [Streptomyces maritimus],6FYC_B The crystal structure of EncM L144M mutant complex with dioxygen under 15 bars O2 pressure [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 1 | 456 | 1 | 457 | Cytochrome P450 monooxygenase cheE OS=Chaetomium globosum (strain ATCC 6205 / CBS 148.51 / DSM 1962 / NBRC 6347 / NRRL 1970) OX=306901 GN=cheE PE=2 SV=1 |
|
| 0.0 | 501 | 1090 | 26 | 616 | FAD-linked oxidoreductase cheF OS=Chaetomium globosum (strain ATCC 6205 / CBS 148.51 / DSM 1962 / NBRC 6347 / NRRL 1970) OX=306901 GN=cheF PE=2 SV=1 |
|
| 3.44e-182 | 515 | 1086 | 2 | 580 | FAD-linked oxidoreductase OXR2 OS=Magnaporthe oryzae (strain 70-15 / ATCC MYA-4617 / FGSC 8958) OX=242507 GN=OXR2 PE=2 SV=1 |
|
| 1.16e-126 | 532 | 1084 | 15 | 611 | FAD-linked oxidoreductase ffsJ OS=Aspergillus flavipes OX=41900 GN=ffsJ PE=3 SV=1 |
|
| 1.85e-96 | 31 | 456 | 23 | 445 | Cytochrome P450 monooxygenase ccsG OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=ccsG PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000057 | 0.000001 |