You are browsing environment: FUNGIDB
CAZyme Information: CXQ87_002197-t45_1-p1
You are here: Home > Sequence: CXQ87_002197-t45_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | [Candida] duobushaemulonis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Saccharomycetes; ; Debaryomycetaceae; Candida; [Candida] duobushaemulonis | |||||||||||
| CAZyme ID | CXQ87_002197-t45_1-p1 | |||||||||||
| CAZy Family | GH5 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH72 | 27 | 239 | 3.1e-82 | 0.6762820512820513 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 397351 | Glyco_hydro_72 | 6.97e-109 | 27 | 311 | 11 | 308 | Glucanosyltransferase. This is a family of glycosylphosphatidylinositol-anchored beta(1-3)glucanosyltransferases. The active site residues in the Aspergillus fumigatus example are the two glutamate residues at 160 and 261. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 21 | 536 | 927 | 1457 | |
| 1.65e-150 | 23 | 422 | 27 | 468 | |
| 1.65e-150 | 23 | 422 | 27 | 468 | |
| 1.06e-148 | 23 | 422 | 27 | 468 | |
| 1.43e-146 | 23 | 422 | 27 | 468 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.52e-102 | 28 | 420 | 36 | 453 | Saccharomyces cerevisiae Gas2p in complex with laminaripentaose [Saccharomyces cerevisiae],2W63_A Saccharomyces Cerevisiae Gas2p In Complex With Laminaritriose And Laminaritetraose [Saccharomyces cerevisiae],5O9O_A Crystal structure of ScGas2 in complex with compound 7. [Saccharomyces cerevisiae S288C],5O9P_A Crystal structure of Gas2 in complex with compound 10 [Saccharomyces cerevisiae S288C],5O9Q_A Crystal structure of ScGas2 in complex with compound 6 [Saccharomyces cerevisiae S288C],5O9R_A Crystal structure of ScGas2 in complex with compound 9 [Saccharomyces cerevisiae S288C],5O9Y_A Crystal structure of ScGas2 in complex with compound 11 [Saccharomyces cerevisiae S288C],5OA2_A Crystal structure of ScGas2 in complex with compound 8 [Saccharomyces cerevisiae S288C],5OA2_B Crystal structure of ScGas2 in complex with compound 8 [Saccharomyces cerevisiae S288C],5OA2_C Crystal structure of ScGas2 in complex with compound 8 [Saccharomyces cerevisiae S288C],5OA6_A Crystal structure of ScGas2 in complex with compound 12 [Saccharomyces cerevisiae S288C] |
|
| 7.05e-102 | 28 | 420 | 36 | 453 | Saccharomyces cerevisiae Gas2p apostructure (E176Q mutant) [Saccharomyces cerevisiae] |
|
| 7.05e-102 | 28 | 420 | 36 | 453 | SACCHAROMYCES CEREVISIAE GAS2P (E176Q MUTANT) IN COMPLEX WITH LAMINARITETRAOSE AND LAMINARIPENTAOSE [Saccharomyces cerevisiae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.30e-101 | 28 | 420 | 36 | 453 | 1,3-beta-glucanosyltransferase GAS2 OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=GAS2 PE=1 SV=1 |
|
| 5.50e-99 | 28 | 419 | 55 | 484 | 1,3-beta-glucanosyltransferase PGA5 OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=PGA5 PE=1 SV=2 |
|
| 1.04e-98 | 11 | 419 | 11 | 430 | Protein EPD1 OS=Candida maltosa OX=5479 GN=EPD1 PE=3 SV=1 |
|
| 8.84e-97 | 27 | 419 | 28 | 442 | Protein EPD2 OS=Candida maltosa OX=5479 GN=EPD2 PE=3 SV=1 |
|
| 1.21e-96 | 8 | 440 | 7 | 474 | pH-responsive protein 1 OS=Candida albicans (strain SC5314 / ATCC MYA-2876) OX=237561 GN=PHR1 PE=2 SV=4 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
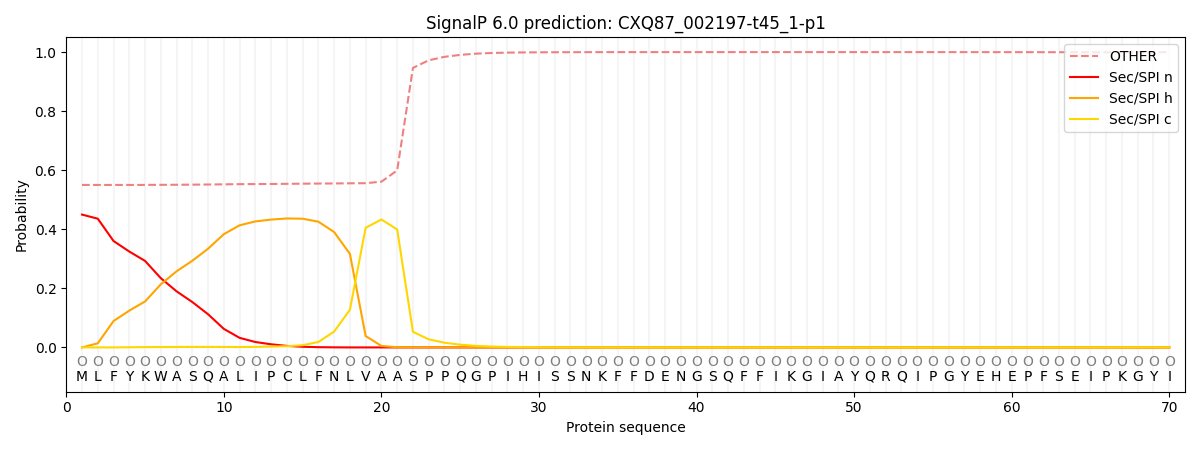
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.562110 | 0.437881 |
