You are browsing environment: FUNGIDB
CAZyme Information: CVK97613.1
You are here: Home > Sequence: CVK97613.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Fusarium mangiferae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Sordariomycetes; ; Nectriaceae; Fusarium; Fusarium mangiferae | |||||||||||
| CAZyme ID | CVK97613.1 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | Amb_all domain-containing protein [Source:UniProtKB/TrEMBL;Acc:A0A1L7TN66] | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 80 | 253 | 3e-107 | 0.9943181818181818 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 214765 | Amb_all | 1.24e-56 | 83 | 252 | 12 | 186 | Amb_all domain. |
| 226384 | PelB | 8.80e-51 | 56 | 335 | 62 | 344 | Pectate lyase [Carbohydrate transport and metabolism]. |
| 366158 | Pec_lyase_C | 2.41e-34 | 85 | 252 | 32 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 1.76e-256 | 1 | 336 | 1 | 336 | |
| 1.05e-252 | 1 | 336 | 1 | 336 | |
| 1.07e-248 | 1 | 336 | 1 | 339 | |
| 7.70e-239 | 21 | 336 | 6 | 324 | |
| 2.50e-231 | 1 | 336 | 1 | 336 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.21e-39 | 29 | 335 | 3 | 325 | Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
|
| 1.80e-32 | 85 | 335 | 130 | 415 | Structure of the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
|
| 2.57e-29 | 42 | 335 | 21 | 331 | Catalytic function and substrate recognition of the pectate lyase from Thermotoga maritima [Thermotoga maritima] |
|
| 7.33e-26 | 85 | 230 | 125 | 296 | Structural insights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
|
| 9.24e-26 | 85 | 230 | 146 | 317 | Bacillus Subtilis Pectate Lyase [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 9.10e-56 | 32 | 316 | 44 | 307 | Pectate lyase A OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=plyA PE=1 SV=1 |
|
| 2.62e-55 | 9 | 335 | 14 | 339 | Pectate trisaccharide-lyase OS=Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46) OX=279010 GN=BLi04129 PE=3 SV=1 |
|
| 2.62e-55 | 9 | 335 | 14 | 339 | Pectate trisaccharide-lyase OS=Bacillus sp. OX=1409 GN=pel PE=1 SV=1 |
|
| 2.62e-55 | 9 | 335 | 14 | 339 | Pectate trisaccharide-lyase OS=Bacillus licheniformis OX=1402 GN=pelA PE=1 SV=1 |
|
| 3.48e-55 | 1 | 260 | 1 | 266 | Probable pectate lyase B OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=plyB PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
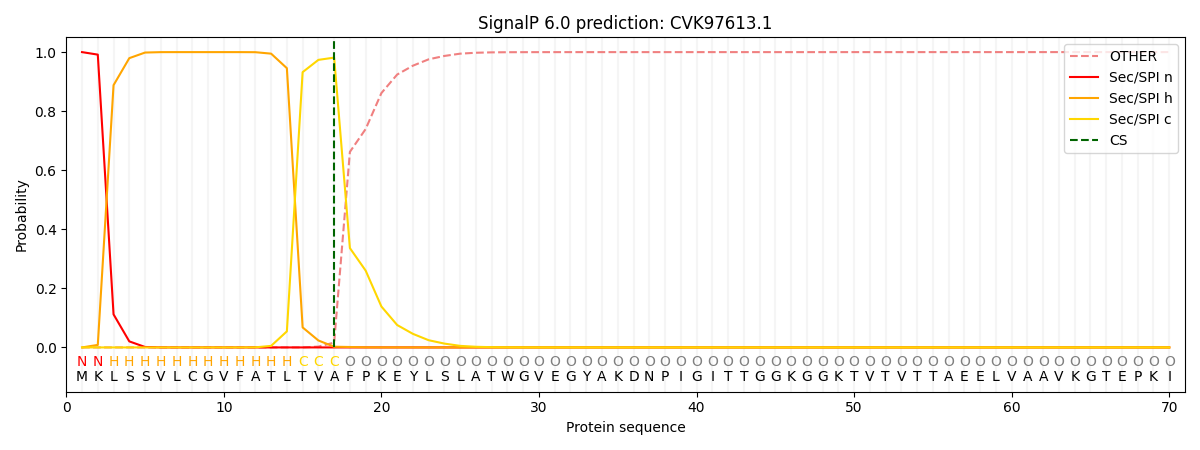
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000237 | 0.999726 | CS pos: 17-18. Pr: 0.9813 |
