You are browsing environment: FUNGIDB
CAZyme Information: CNBC4720-t26_1-p1
You are here: Home > Sequence: CNBC4720-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Cryptococcus neoformans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Arthropoda; Insecta; ; Eriococcidae; Cryptococcus; Cryptococcus neoformans | |||||||||||
| CAZyme ID | CNBC4720-t26_1-p1 | |||||||||||
| CAZy Family | GH128 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.21:16 | 3.2.1.-:3 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH3 | 130 | 331 | 7.2e-61 | 0.9583333333333334 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396478 | Glyco_hydro_3_C | 3.25e-56 | 417 | 651 | 1 | 216 | Glycosyl hydrolase family 3 C-terminal domain. This domain is involved in catalysis and may be involved in binding beta-glucan. This domain is found associated with pfam00933. |
| 224389 | BglX | 1.11e-50 | 143 | 431 | 75 | 352 | Periplasmic beta-glucosidase and related glycosidases [Carbohydrate transport and metabolism]. |
| 185053 | PRK15098 | 9.43e-50 | 100 | 814 | 46 | 762 | beta-glucosidase BglX. |
| 395747 | Glyco_hydro_3 | 2.37e-35 | 151 | 368 | 88 | 313 | Glycosyl hydrolase family 3 N terminal domain. |
| 178629 | PLN03080 | 5.53e-33 | 90 | 772 | 51 | 740 | Probable beta-xylosidase; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 819 | 1 | 819 | |
| 0.0 | 1 | 818 | 1 | 814 | |
| 0.0 | 1 | 818 | 1 | 814 | |
| 0.0 | 1 | 818 | 1 | 814 | |
| 0.0 | 1 | 818 | 2 | 819 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 8.05e-238 | 78 | 804 | 12 | 826 | Chain A, Beta-glucosidase [Thermochaetoides thermophila] |
|
| 1.96e-227 | 78 | 804 | 33 | 842 | Chain A, Beta-glucosidase [Rasamsonia emersonii],5JU6_B Chain B, Beta-glucosidase [Rasamsonia emersonii],5JU6_C Chain C, Beta-glucosidase [Rasamsonia emersonii],5JU6_D Chain D, Beta-glucosidase [Rasamsonia emersonii] |
|
| 6.63e-227 | 78 | 804 | 14 | 828 | Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus [Aspergillus aculeatus],4IIB_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus [Aspergillus aculeatus],4IIC_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with isofagomine [Aspergillus aculeatus],4IIC_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with isofagomine [Aspergillus aculeatus],4IID_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with 1-deoxynojirimycin [Aspergillus aculeatus],4IID_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with 1-deoxynojirimycin [Aspergillus aculeatus],4IIE_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with calystegine B(2) [Aspergillus aculeatus],4IIE_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with calystegine B(2) [Aspergillus aculeatus],4IIF_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with castanospermine [Aspergillus aculeatus],4IIF_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with castanospermine [Aspergillus aculeatus],4IIG_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with D-glucose [Aspergillus aculeatus],4IIG_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with D-glucose [Aspergillus aculeatus],4IIH_A Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with thiocellobiose [Aspergillus aculeatus],4IIH_B Crystal structure of beta-glucosidase 1 from Aspergillus aculeatus in complex with thiocellobiose [Aspergillus aculeatus] |
|
| 3.50e-224 | 84 | 804 | 21 | 829 | Three-dimensional structures of two heavily N-glycosylated Aspergillus sp. Family GH3 beta-D-glucosidases [Aspergillus oryzae],5FJJ_B Three-dimensional structures of two heavily N-glycosylated Aspergillus sp. Family GH3 beta-D-glucosidases [Aspergillus oryzae],5FJJ_C Three-dimensional structures of two heavily N-glycosylated Aspergillus sp. Family GH3 beta-D-glucosidases [Aspergillus oryzae],5FJJ_D Three-dimensional structures of two heavily N-glycosylated Aspergillus sp. Family GH3 beta-D-glucosidases [Aspergillus oryzae] |
|
| 2.26e-223 | 55 | 808 | 9 | 851 | Structural studies of a Glycoside Hydrolase Family 3 beta-glucosidase from the Model Fungus Neurospora crassa [Neurospora crassa OR74A],5NBS_B Structural studies of a Glycoside Hydrolase Family 3 beta-glucosidase from the Model Fungus Neurospora crassa [Neurospora crassa OR74A] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.03e-247 | 65 | 806 | 5 | 726 | Probable beta-glucosidase L OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=bglL PE=3 SV=1 |
|
| 4.03e-247 | 65 | 806 | 5 | 726 | Probable beta-glucosidase L OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=bglL PE=3 SV=1 |
|
| 4.03e-247 | 65 | 806 | 5 | 726 | Probable beta-glucosidase L OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=bglL PE=3 SV=1 |
|
| 9.13e-240 | 85 | 806 | 25 | 727 | Probable beta-glucosidase L OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=bglL PE=3 SV=1 |
|
| 3.78e-231 | 85 | 806 | 26 | 728 | Probable beta-glucosidase L OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=bglL PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
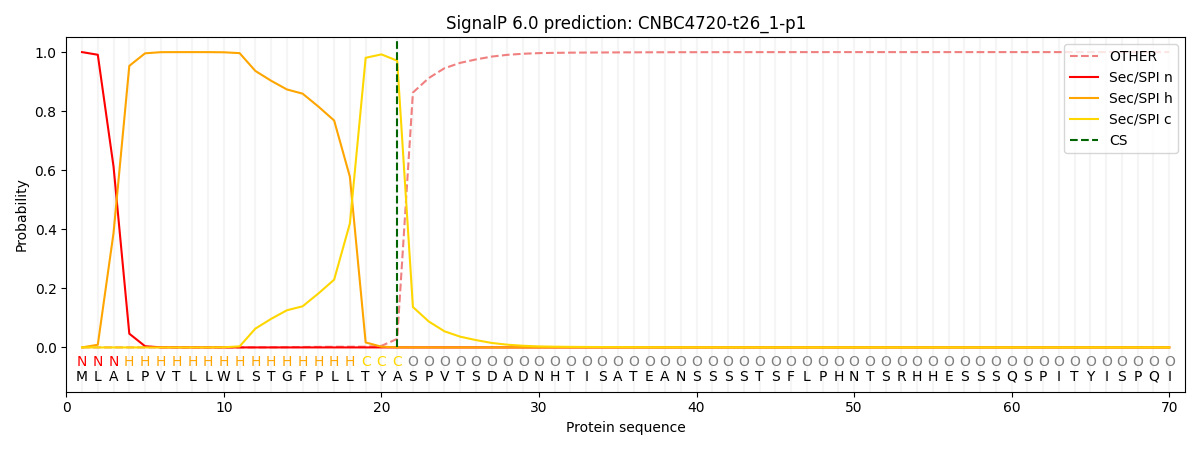
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000491 | 0.999499 | CS pos: 21-22. Pr: 0.9710 |
