You are browsing environment: FUNGIDB
CAZyme Information: CDH59937.1
You are here: Home > Sequence: CDH59937.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Lichtheimia corymbifera | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Mucoromycota; Mucoromycetes; ; Lichtheimiaceae; Lichtheimia; Lichtheimia corymbifera | |||||||||||
| CAZyme ID | CDH59937.1 | |||||||||||
| CAZy Family | GT49 | |||||||||||
| CAZyme Description | l-ascorbate oxidase-like | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 44 | 540 | 1.9e-98 | 0.9692737430167597 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 132431 | ascorbOXfungal | 3.74e-80 | 27 | 543 | 13 | 528 | L-ascorbate oxidase, fungal type. This model describes a family of fungal ascorbate oxidases, within a larger family of multicopper oxidases that also includes plant ascorbate oxidases (TIGR03388), plant laccases and laccase-like proteins (TIGR03389), and related proteins. The member from Acremonium sp. HI-25 is characterized. |
| 274555 | ascorbase | 8.62e-73 | 23 | 551 | 2 | 528 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
| 215324 | PLN02604 | 4.70e-65 | 19 | 545 | 21 | 545 | oxidoreductase |
| 177843 | PLN02191 | 8.28e-65 | 20 | 551 | 21 | 551 | L-ascorbate oxidase |
| 274556 | laccase | 4.86e-56 | 46 | 546 | 25 | 519 | laccase, plant. Members of this protein family include the copper-containing enzyme laccase (EC 1.10.3.2), often several from a single plant species, and additional, uncharacterized, closely related plant proteins termed laccase-like multicopper oxidases. This protein family shows considerable sequence similarity to the L-ascorbate oxidase (EC 1.10.3.3) family. Laccases are enzymes of rather broad specificity, and classification of all proteins scoring about the trusted cutoff of this model as laccases may be appropriate. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 522 | 1 | 523 | |
| 2.92e-164 | 21 | 551 | 26 | 573 | |
| 8.26e-126 | 21 | 551 | 24 | 645 | |
| 9.66e-124 | 24 | 551 | 17 | 570 | |
| 1.29e-62 | 7 | 551 | 11 | 551 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.25e-59 | 48 | 551 | 27 | 528 | Refined Crystal Structure Of Ascorbate Oxidase At 1.9 Angstroms Resolution [Cucurbita pepo var. melopepo],1AOZ_B Refined Crystal Structure Of Ascorbate Oxidase At 1.9 Angstroms Resolution [Cucurbita pepo var. melopepo],1ASO_A X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASO_B X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASP_A X-ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-forms [Cucurbita pepo var. melopepo],1ASP_B X-ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-forms [Cucurbita pepo var. melopepo],1ASQ_A X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo],1ASQ_B X-Ray Structures And Mechanistic Implications Of Three Functional Derivatives Of Ascorbate Oxidase From Zucchini: Reduced-, Peroxide-, And Azide-Forms [Cucurbita pepo var. melopepo] |
|
| 4.52e-43 | 46 | 552 | 25 | 536 | Crystal Structure of the Zea Mays laccase 3 [Zea mays],6KLI_A Crystal Structure of the Zea Mays laccase 3 complexed with sinapyl [Zea mays],6KLJ_A Crystal Structure of the Zea Mays laccase 3 complexed with coniferyl [Zea mays] |
|
| 3.28e-40 | 27 | 543 | 9 | 465 | Chain A, LACCASE 1 [Coprinopsis cinerea] |
|
| 3.33e-40 | 27 | 543 | 9 | 465 | Chain A, Laccase [Coprinopsis cinerea] |
|
| 1.45e-39 | 26 | 549 | 7 | 471 | CRYSTAL STRUCTURE OF A FULLY FUNCTIONAL LACCASE FROM THE LIGNINOLYTIC FUNGUS PYCNOPORUS CINNABARINUS [Trametes cinnabarina] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.57e-59 | 7 | 543 | 18 | 556 | L-ascorbate oxidase OS=Cucumis sativus OX=3659 PE=1 SV=1 |
|
| 1.67e-58 | 48 | 551 | 27 | 528 | L-ascorbate oxidase OS=Cucurbita pepo var. melopepo OX=3665 PE=1 SV=1 |
|
| 7.46e-57 | 48 | 551 | 57 | 558 | L-ascorbate oxidase OS=Cucurbita maxima OX=3661 GN=AAO PE=1 SV=2 |
|
| 1.30e-56 | 27 | 547 | 35 | 594 | Laccase-like multicopper oxidase 1 OS=Myceliophthora thermophila (strain ATCC 42464 / BCRC 31852 / DSM 1799) OX=573729 GN=LMCO1 PE=1 SV=1 |
|
| 8.51e-55 | 8 | 551 | 6 | 547 | L-ascorbate oxidase OS=Brassica rapa subsp. pekinensis OX=51351 GN=AO PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
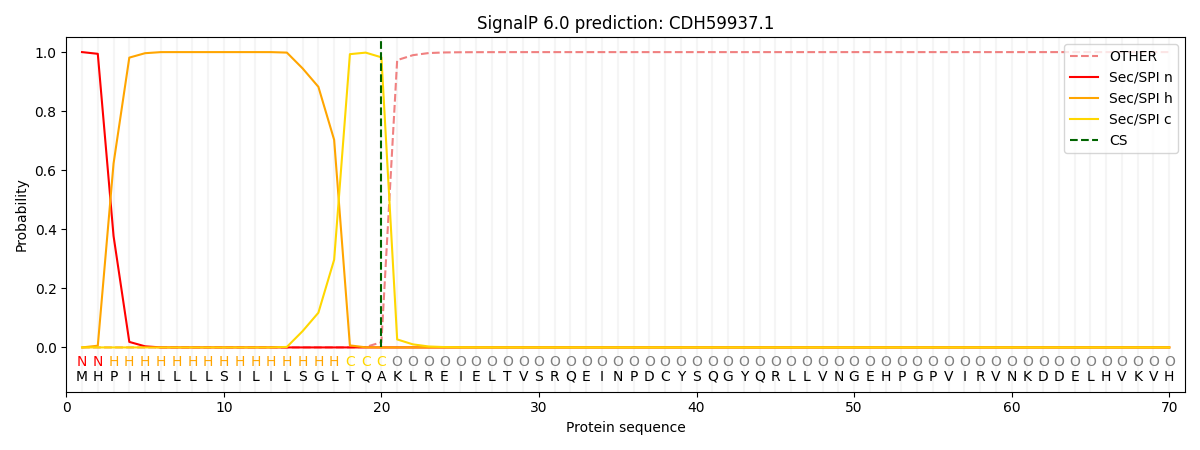
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000199 | 0.999781 | CS pos: 20-21. Pr: 0.9823 |
