You are browsing environment: FUNGIDB
CAZyme Information: CCG84461.1
You are here: Home > Sequence: CCG84461.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Taphrina deformans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Taphrinomycetes; ; Taphrinaceae; Taphrina; Taphrina deformans | |||||||||||
| CAZyme ID | CCG84461.1 | |||||||||||
| CAZy Family | GT25 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH152 | 37 | 162 | 1.3e-31 | 0.5833333333333334 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395248 | Thaumatin | 3.27e-34 | 37 | 162 | 1 | 123 | Thaumatin family. |
| 185757 | TLP-PA | 1.16e-31 | 32 | 165 | 1 | 130 | allergenic/antifungal thaumatin-like proteins: plant and animal homologs. This subfamily is represented by the thaumatin-like proteins (TLPs), Cherry Allergen Pru Av 2 TLP, Peach PpAZ44 TLP (a propylene-induced TLP in abscission), the Caenorhabditis elegans thaumatin family member (thn-6), and other plant and animal homologs. TLPs are involved in host defense and a wide range of developmental processes in fungi, plants, and animals. Due to their inducible expression by environmental stresses such as pathogen/pest attack, drought and cold, plant TLPs are classified as the pathogenesis-related (PR) protein family 5 (PR5). Several members of the plant TLP family have been reported as food allergens from fruits (i.e., cherry, Pru av 2; bell pepper, Cap a1; tomatoes, Lyc e NP24) and pollen allergens from conifers (i.e., mountain cedar, Jun a 3; Arizona cypress, Cup a3; Japanese cedar, Cry j3). TLPs are three-domain, crescent-fold structures with either an electronegative, electropositive, or neutral cleft occurring between domains I and II. It has been proposed that the antifungal activity of plant PR5 proteins relies on the strong electronegative character of this cleft. Some TLPs hydrolyze the beta-1,3-glucans of the type commonly found in fungal walls. TLPs within this subfamily contain 16 conserved Cys residues. |
| 185754 | Thaumatin-like | 1.54e-28 | 33 | 161 | 1 | 127 | the sweet-tasting protein, thaumatin, and thaumatin-like proteins involved in host defense. This family is represented by the sweet-tasting protein thaumatin from the African berry Thaumatococcus daniellii and thaumatin-like proteins (TLPs) involved in host defense and a wide range of developmental processes in fungi, plants, and animals. Plant TLPs are classified as pathogenesis-related (PR) protein family 5 (PR5), their expression is induced by environmental stresses such as pathogen/pest attack, drought and cold. TLPs included in this family are such proteins as zeamatin, found in high concentrations in cereal seeds; osmotin, a salt-induced protein in osmotically stressed plants; and PpAZ44, a propylene-induced TLP in abscission of young fruit. Several members of the plant TLP family have been reported as food allergens from fruits (i.e., cherry, Pru av 2; bell pepper, Cap a1; tomatoes, Lyc e NP24) and pollen allergens from conifers (i.e., mountain cedar, Jun a 3; Arizona cypress, Cup a3; Japanese cedar, Cry j3). Thaumatin and TLPs are three-domain, crescent-fold structures with either an electronegative, electropositive, or neutral cleft occurring between domains I and II. It has been proposed that the antifungal activity of plant PR5 proteins relies on the strong electronegative character of this cleft. Some TLPs hydrolyze the beta-1,3-glucans of the type commonly found in fungal walls. Most TLPs contain 16 conserved Cys residues. A deletion within the third domain (domain II) of the Triticum aestivum thaumatin-like xylanase inhibitor is observed, thus, only 10 conserved Cys residues are present within this smaller TLP and similar homologs. |
| 185758 | TLP-F | 3.87e-26 | 33 | 161 | 1 | 130 | thaumatin-like proteins: basidiomycete homologs. This subfamily is represented by Lentinula edodes TLG1, a thaumatin-like protein (TLP), as well as, other basidiomycete homologs. In general, TLPs are involved in host defense and a wide range of developmental processes in fungi, plants, and animals. TLG1 TLP is involved in lentinan degradation and fruiting body senescence. TLG1 expressed in Escherichia coli and Aspergillus oryzae exhibited beta-1,3-glucanase activity and demonstrated lentinan degrading activity. TLG1 is proposed to be involved in lentinan and cell wall degradation during senescence following harvest and spore diffusion. TLPs are three-domain, crescent-fold structures with either an electronegative, electropositive, or neutral cleft occurring between domains I and II. TLG1 from Lentinula edodes contains the required acidic amino acids conserved in the appropriate positions to possess an electronegative cleft. TLPs within this subfamily contain 13 conserved Cys residues; the number of total Cys residues in these TLPs varies from 16 in L. edodes TLG1 to 18 in other basidiomycete homologs. |
| 128501 | THN | 1.60e-25 | 33 | 161 | 1 | 130 | Thaumatin family. The thaumatin family gathers proteins related to plant pathogenesis. The thaumatin family includes very basic members with extracellular and vacuolar localization. Thaumatin itsel is a potent sweet-tasting protein. Several members of this family display significant in vitro activity of inhibiting hyphal growth or spore germination of various fungi probably by a membrane permeabilizing mechanism. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.23e-23 | 32 | 154 | 22 | 145 | |
| 1.73e-22 | 33 | 160 | 23 | 151 | |
| 3.09e-22 | 33 | 160 | 23 | 151 | |
| 3.49e-21 | 33 | 160 | 23 | 151 | |
| 4.39e-21 | 32 | 195 | 22 | 173 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.11e-14 | 33 | 161 | 3 | 133 | High resolution structure of Mal d 2, the thaumatin like food allergen from apple [Malus domestica] |
|
| 2.28e-14 | 32 | 167 | 2 | 147 | Thaumatin Before A High Dose X-Ray 'Burn' [Thaumatococcus daniellii],2BLU_A Thaumatin After A High Dose X-Ray 'Burn' [Thaumatococcus daniellii],2VHK_A Atomic resolution (0.94 A) structure of purified thaumatin I grown in sodium L-tartrate at 22C [Thaumatococcus daniellii],2WBZ_A 1.6 A Structure of Thaumatin Crystallized without Tartrate at 4 C [Thaumatococcus daniellii],3N02_A Thaumatic crystals grown in loops/micromounts [Thaumatococcus daniellii],3N03_A Thaumatin crystals grown from drops [Thaumatococcus daniellii],4AXU_A CRYSTAL STRUCTURE OF THAUMATIN FROM AN AUTO-HARVESTED CRYSTAL, control experiment [Thaumatococcus daniellii],5AMZ_A Crystal Structure of Thaumatin processed with the CrystalDirect automated mounting and cryo-cooling technology [Thaumatococcus daniellii],5AVG_A The 0.95 angstrom structure of thaumatin crystallized in high-strength agarose hydrogel [Thaumatococcus daniellii] |
|
| 2.31e-14 | 32 | 167 | 2 | 147 | Crystal structure of thaumatin at high hydrostatic pressure [Thaumatococcus daniellii],1LR3_A Crystal structure of thaumatin at high hydrostatic pressure [Thaumatococcus daniellii],1LXZ_A Structure of thaumatin crystallized in the presence of glycerol [Thaumatococcus daniellii],1LY0_A Structure of thaumatin crystallized in the presence of glycerol [Thaumatococcus daniellii],1PP3_A Structure of thaumatin in a hexagonal space group [Thaumatococcus daniellii],1PP3_B Structure of thaumatin in a hexagonal space group [Thaumatococcus daniellii],1THI_A Crystal Structures Of Two Intensely Sweet Proteins [Thaumatococcus daniellii],2OQN_A High Pressure Cryocooling of Capillary Sample Cryoprotection and Diffraction Phasing at Long Wavelengths [Thaumatococcus daniellii],2VHR_A Atomic resolution (0.95A) structure of purified thaumatin I grown in sodium L-tartrate at 4 C [Thaumatococcus daniellii],2VI1_A Atomic resolution (1.04 A) structure of purified thaumatin I grown in sodium D-tartrate at 22 C. [Thaumatococcus daniellii],2VI2_A Atomic resolution (1.05 A) structure of purified Thaumatin I grown in sodium D-tartrate at 4C [Thaumatococcus daniellii],2VI3_A Atomic resolution (0.98 A) structure of purified thaumatin I grown in sodium DL-tartrate at 20 C [Thaumatococcus daniellii],2VI4_A Atomic resolution (1.10 A) structure of purified thaumatin I grown in sodium DL-tartrate at 6 C. [Thaumatococcus daniellii],2VU6_A Atomic resolution (0.95 A) structure of purified Thaumatin I grown in sodium meso-tartrate at 19 C. [Thaumatococcus daniellii],2VU7_A Atomic resolution (1.08 A) structure of purified thaumatin I grown in sodium meso-tartrate at 4 C [Thaumatococcus daniellii],3QY5_A Microfluidic crystallization of Thaumatin using the Crystal Former [Thaumatococcus daniellii],3ZEJ_A Thaumatin structure determined at room temperature by in-situ diffraction in ChipX [Thaumatococcus daniellii],4AXR_A CRYSTAL STRUCTURE OF thaumatin FROM A AUTO-HARVESTED CRYSTAL [Thaumatococcus daniellii],4BAL_A Thaumatin from Thaumatococcus daniellii structure in complex with the europium tris-hydroxymethyltriazoledipicolinate complex at 1.30 A resolution. [Thaumatococcus daniellii],4BAR_A Thaumatin from Thaumatococcus daniellii structure in complex with the europium tris-hydroxyethyltriazoledipicolinate complex at 1.20 A resolution. [Thaumatococcus daniellii],4C3C_A Thaumatin refined against hatrx data for time-point 1 [Thaumatococcus daniellii],4TVT_A New ligand for thaumatin discovered using acoustic high throughput screening [Thaumatococcus daniellii],4ZG3_A In-vacuum long-wavelength crystallography [Thaumatococcus daniellii],4ZXR_A Structure of Thaumatin wrapped in graphene within vacuum [Thaumatococcus daniellii],5A47_A Structure of Thaumatin obtained by multi crystal data collection [Thaumatococcus daniellii],5FGT_A Thaumatin solved by native sulphur-SAD using free-electron laser radiation [Thaumatococcus daniellii],5FGX_A Thaumatin solved by native sulphur SAD using synchrotron radiation [Thaumatococcus daniellii],5JVX_A X-ray structure of the adduct formed in the reaction between thaumatin and a gold carbene compound [Thaumatococcus daniellii],5K7Q_A MicroED structure of thaumatin at 2.5 A resolution [Thaumatococcus daniellii],5KVW_A T. danielli thaumatin at 100K, Data set 1 [Thaumatococcus daniellii],5KVX_A T. danielli thaumatin at 100K, Data set 2 [Thaumatococcus daniellii],5KVZ_A T. danielli thaumatin at 100K, Data set 3 [Thaumatococcus daniellii],5KW0_A T. danielli thaumatin at 100K, Data set 5 [Thaumatococcus daniellii],5KW3_A T. danielli thaumatin at 278K, Data set 1 [Thaumatococcus daniellii],5KW4_A T. danielli thaumatin at 278K, Data set 2 [Thaumatococcus daniellii],5KW5_A T. danielli thaumatin at 278K, Data set 3 [Thaumatococcus daniellii],5KW7_A T. danielli thaumatin at 278K, Data set 4 [Thaumatococcus daniellii],5KW8_A T. danielli thaumatin at 278K, Data set 5 [Thaumatococcus daniellii],5L4R_A X-ray structure of the adduct between thaumatin and cisplatin [Thaumatococcus daniellii],5LH0_A Low dose Thaumatin - 0-40 ms. [Thaumatococcus daniellii],5LH1_A Low dose Thaumatin - 360-400 ms. [Thaumatococcus daniellii],5LH3_A High dose Thaumatin - 0-40 ms. [Thaumatococcus daniellii],5LH5_A High dose Thaumatin - 40-80 ms. [Thaumatococcus daniellii],5LH6_A High dose Thaumatin - 360-400 ms. [Thaumatococcus daniellii],5LH7_A High dose Thaumatin - 760-800 ms. [Thaumatococcus daniellii],5LMH_A High dose Thaumatin - 160-200 ms. [Thaumatococcus daniellii],5LN0_A Low dose Thaumatin - 760-800 ms. [Thaumatococcus daniellii],5MJG_A Single-shot pink beam serial crystallography: Thaumatin [Thaumatococcus daniellii],5T3G_A thaumatin soaked with selenourea for 10 min [Thaumatococcus daniellii],5TCL_A Thaumatin from 4.96 A wavelength data collection [Thaumatococcus daniellii],6C5Y_A Crystal structure of thaumatin from microcrystals [Thaumatococcus daniellii],6COA_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],6FJ6_A Structure of Thaumatin collected at 100K on ID30B [Thaumatococcus daniellii],6FJ8_A Structure of Thaumatin collected from an in situ crystal collected on ID30B at 12.7 keV. [Thaumatococcus daniellii],6FJ9_A Structure of Thaumatin collected from an in situ crystal on ID30B at 17.5 keV. [Thaumatococcus daniellii],6O8A_A Thaumatin native-SAD structure determined at 5 keV from microcrystals [Thaumatococcus daniellii],6S19_A Structure of thaumatin determined at SwissFEL using native-SAD at 4.57 keV from all available diffraction patterns [Thaumatococcus daniellii],6S1D_A Structure of thaumatin determined at SwissFEL using native-SAD at 4.57 keV from 20,000 diffraction patterns [Thaumatococcus daniellii],6S1E_A Structure of thaumatin determined at SwissFEL using native-SAD at 6.06 keV from all available diffraction patterns [Thaumatococcus daniellii],6S1G_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],6SRJ_A X-ray pump X-ray probe on thaumatin nanocrystals: single pulse reference data [Thaumatococcus daniellii],6SRK_A X-ray pump X-ray probe on thaumatin nanocrystals: 35 fs time delay [Thaumatococcus daniellii],6SRL_A X-ray pump X-ray probe on thaumatin nanocrystals: 54 fs time delay [Thaumatococcus daniellii],6SRO_A X-ray pump X-ray probe on thaumatin nanocrystals: 76 fs time delay [Thaumatococcus daniellii],6SRP_A X-ray pump X-ray probe on thaumatin nanocrystals: 100 fs time delay [Thaumatococcus daniellii],6SRQ_A X-ray pump X-ray probe on thaumatin nanocrystals: 18 fs time delay [Thaumatococcus daniellii],6YBX_A RT structure of Thaumatin obtained at 1.14 A resolution from crystal grown in a Mylar microchip. [Thaumatococcus daniellii],6YC5_A RT structure of Thaumatin obtained at 1.35 A resolution from crystal grown in a Kapton microchip. [Thaumatococcus daniellii],6ZHN_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7AC3_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7O44_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7O51_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7O53_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7O5J_A Chain A, Thaumatin-1 [Thaumatococcus daniellii],7O5K_A Chain A, Thaumatin-1 [Thaumatococcus daniellii] |
|
| 2.31e-14 | 32 | 167 | 2 | 147 | Structure of VIL-thaumatin [Thaumatococcus daniellii] |
|
| 3.22e-14 | 32 | 167 | 2 | 147 | Structure K106A thaumatin [Thaumatococcus daniellii] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.30e-23 | 32 | 195 | 22 | 173 | Pathogenesis-related protein 1C OS=Hordeum vulgare OX=4513 PE=2 SV=1 |
|
| 1.30e-23 | 32 | 195 | 22 | 173 | Pathogenesis-related protein 1A/1B OS=Hordeum vulgare OX=4513 PE=2 SV=1 |
|
| 7.80e-22 | 32 | 195 | 22 | 173 | Thaumatin-like protein PWIR2 OS=Triticum aestivum OX=4565 PE=2 SV=1 |
|
| 2.47e-18 | 32 | 195 | 23 | 169 | Thaumatin-like pathogenesis-related protein 3 OS=Avena sativa OX=4498 GN=RASTL-3 PE=2 SV=1 |
|
| 5.71e-18 | 33 | 161 | 27 | 157 | Thaumatin-like protein 1 OS=Prunus persica OX=3760 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
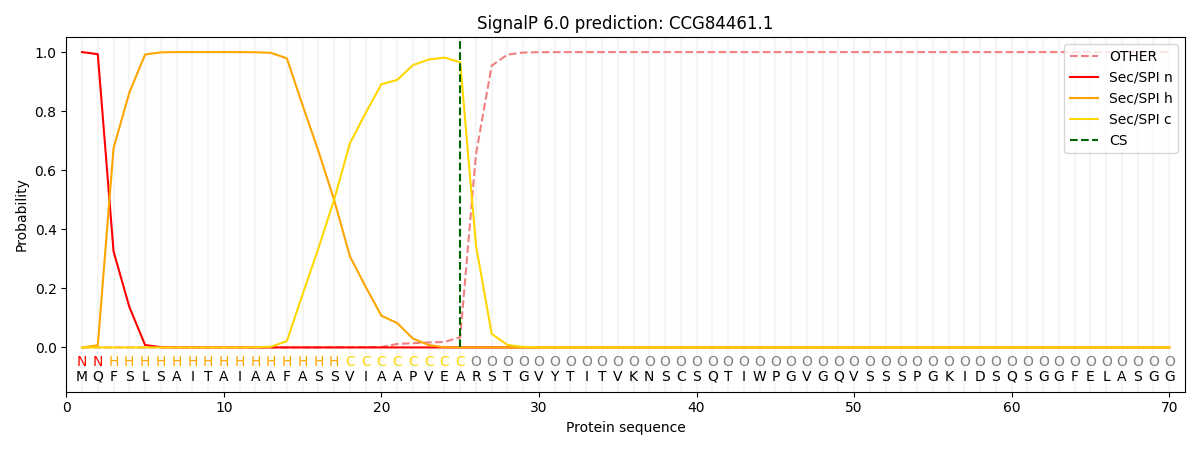
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000203 | 0.999783 | CS pos: 25-26. Pr: 0.9656 |
