You are browsing environment: FUNGIDB
CAZyme Information: CC1G_05304-t26_1-p1
You are here: Home > Sequence: CC1G_05304-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Coprinopsis cinerea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Agaricomycetes; ; Psathyrellaceae; Coprinopsis; Coprinopsis cinerea | |||||||||||
| CAZyme ID | CC1G_05304-t26_1-p1 | |||||||||||
| CAZy Family | CE5 | |||||||||||
| CAZyme Description | rhamnogalacturonase B | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 4.2.2.23:4 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL4 | 20 | 520 | 7.3e-201 | 0.9919839679358717 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 401282 | RhgB_N | 1.45e-134 | 23 | 268 | 1 | 249 | Rhamnogalacturonan lyase B, N-terminal. Members of this family are found in both fungi, bacteria and wood-eating arthropods. The domain is found at the N-terminus of rhamnogalacturonase B, a member of the polysaccharide lyase family 4. The domain adopts a structure consisting of a beta super-sandwich, with eighteen strands in two beta-sheets. The three domains of the whole protein rhamnogalacturonan lyase (RGL4), are involved in the degradation of rhamnogalacturonan-I, RG-I, an important pectic plant cell-wall polysaccharide. The active-site residues are a lysine at position 169 in UniProtKB:Q00019 and a histidine at 229, Lys169 is likely to be a proton abstractor, His229 a proton donor in the mechanism. The substrate is a disaccharide, and RGL4, in contrast to other rhamnogalacturonan hydrolases, cleaves the alpha-1,4 linkages of RG-I between Rha and GalUA through a beta-elimination resulting in a double bond in the nonreducing GalUA residue, and is thus classified as a polysaccharide lyase (PL). |
| 199907 | RGL4_N | 3.13e-52 | 23 | 255 | 1 | 263 | N-terminal catalytic domain of rhamnogalacturonan lyase, a family 4 polysaccharide lyase. The rhamnogalacturonan lyase of the polysaccharide lyase family 4 (RGL4) is involved in the degradation of RG (rhamnogalacturonan) type-I, an important pectic plant cell wall polysaccharide, by cleaving the alpha-1,4 glycoside bond between L-rhamnose and D-galacturonic acids in the backbone of RG type-I through a beta-elimination reaction. RGL4 consists of three domains, an N-terminal catalytic domain, a middle domain with a FNIII type fold and a C-terminal domain with a jelly roll fold; the middle and C-terminal domains are both putative carbohydrate binding modules. There are two types of RG lyases, which both cleave the alpha-1,4 bonds of the RG-I main chain (RG chain) through the beta-elimination reaction, but belong to two structurally unrelated polysaccharide lyase (PL) families, 4 and 11. |
| 199905 | RGL4_C | 8.98e-52 | 365 | 515 | 1 | 148 | C-terminal domain of rhamnogalacturonan lyase, a family 4 polysaccharide lyase. The rhamnogalacturonan lyase of the polysaccharide lyase family 4 (RGL4) is involved in the degradation of RG (rhamnogalacturonan) type-I, an important pectic plant cell wall polysaccharide, by cleaving the alpha-1,4 glycoside bond between L-rhamnose and D-galacturonic acids in the backbone of RG type-I through a beta-elimination reaction. RGL4 consists of three domains, an N-terminal catalytic domain, a middle domain with a FNIII type fold and a C-terminal domain with a jelly roll fold. Both the middle and the C-terminal domain are putative carbohydrate binding modules. There are two types of RG lyases, which both cleave the alpha-1,4 bonds of the RG-I main chain (RG chain) through the beta-elimination reaction, but belong to two structurally unrelated polysaccharide lyase (PL) families, 4 and 11. |
| 405384 | CBM-like | 4.14e-50 | 363 | 520 | 1 | 151 | Polysaccharide lyase family 4, domain III. CBM-like is domain III of rhamnogalacturonan lyase (RG-lyase). The full-length protein specifically recognizes and cleaves alpha-1,4 glycosidic bonds between l-rhamnose and d-galacturonic acids in the backbone of rhamnogalacturonan-I, a major component of the plant cell wall polysaccharide, pectin. This domain possesses a jelly roll beta-sandwich fold structurally homologous to carbohydrate binding modules (CBMs), and it carries two sulfate ions and a hexa-coordinated calcium ion. |
| 405387 | fn3_3 | 8.15e-21 | 276 | 351 | 1 | 74 | Polysaccharide lyase family 4, domain II. FnIII-like is domain II of rhamnogalacturonan lyase (RG-lyase). The full-length protein specifically recognizes and cleaves alpha-1,4 glycosidic bonds between l-rhamnose and d-galacturonic acids in the backbone of rhamnogalacturonan-I, a major component of the plant cell wall polysaccharide, pectin. This domain displays an immunoglobulin-like or more specifically Fibronectin-III type fold and shows highest structural similarity to the C-terminal beta-sandwich subdomain of the pro-hormone/propeptide processing enzyme carboxypeptidase gp180 from duck. It serves to assist in producing the deep pocket, with domain III, into which the substrate fits. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 9.14e-240 | 11 | 520 | 11 | 515 | |
| 3.32e-228 | 9 | 520 | 9 | 516 | |
| 1.35e-227 | 9 | 520 | 9 | 516 | |
| 7.22e-226 | 9 | 520 | 10 | 510 | |
| 7.28e-225 | 9 | 520 | 9 | 516 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.16e-165 | 22 | 520 | 1 | 495 | Rhamnogalacturonan lyase from Aspergillus aculeatus [Aspergillus aculeatus] |
|
| 1.74e-164 | 22 | 520 | 1 | 495 | Rhamnogalacturonan lyase from Aspergillus aculeatus K150A active site mutant [Aspergillus aculeatus],2XHN_B Rhamnogalacturonan lyase from Aspergillus aculeatus K150A active site mutant [Aspergillus aculeatus],3NJV_A Rhamnogalacturonan lyase from Aspergillus aculeatus K150A substrate complex [Aspergillus aculeatus] |
|
| 7.01e-164 | 22 | 520 | 1 | 495 | Rhamnogalacturonan Lyase from Aspergillus aculeatus mutant H210A [Aspergillus aculeatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.01e-173 | 12 | 520 | 11 | 515 | Probable rhamnogalacturonate lyase A OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=rglA PE=3 SV=2 |
|
| 1.11e-169 | 16 | 520 | 15 | 515 | Probable rhamnogalacturonate lyase A OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=rglA PE=3 SV=1 |
|
| 1.11e-169 | 16 | 520 | 15 | 515 | Probable rhamnogalacturonate lyase A OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=rglA PE=3 SV=1 |
|
| 5.64e-167 | 13 | 520 | 11 | 514 | Rhamnogalacturonate lyase A OS=Aspergillus aculeatus OX=5053 GN=rglA PE=1 SV=1 |
|
| 3.80e-165 | 10 | 520 | 9 | 515 | Probable rhamnogalacturonate lyase A OS=Neosartorya fischeri (strain ATCC 1020 / DSM 3700 / CBS 544.65 / FGSC A1164 / JCM 1740 / NRRL 181 / WB 181) OX=331117 GN=rglA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
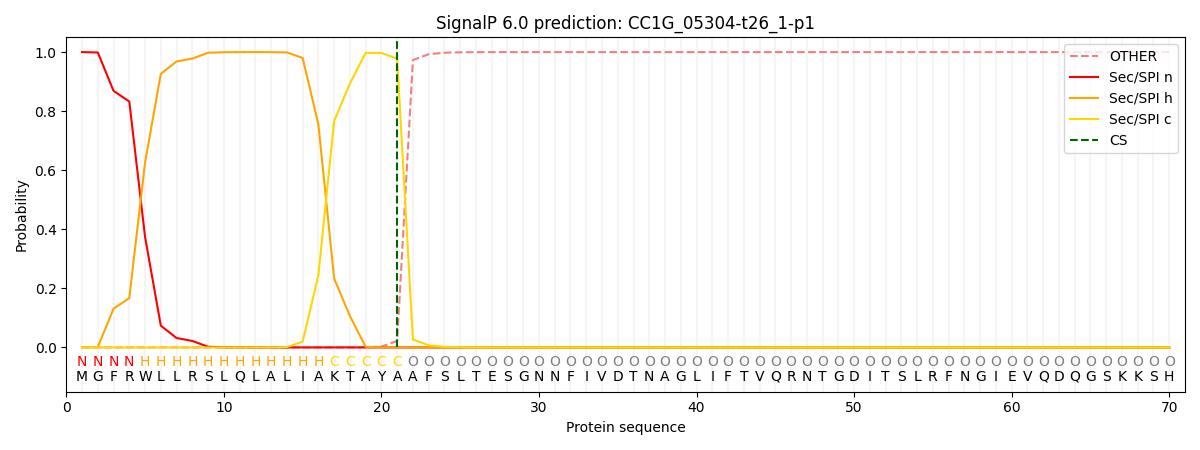
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000329 | 0.999660 | CS pos: 21-22. Pr: 0.9780 |
