You are browsing environment: FUNGIDB
CAZyme Information: CAH01122.1
You are here: Home > Sequence: CAH01122.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Kluyveromyces lactis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Saccharomycetes; ; Saccharomycetaceae; Kluyveromyces; Kluyveromyces lactis | |||||||||||
| CAZyme ID | CAH01122.1 | |||||||||||
| CAZy Family | GT15 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 38 | 514 | 8.4e-82 | 0.9553072625698324 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 259932 | CuRO_1_MCO_like_2 | 9.33e-64 | 4 | 146 | 1 | 139 | The second cupredoxin domain of uncharacterized multicopper oxidase. Multicopper Oxidases (MCOs) are multi-domain enzymes that are able to couple oxidation of substrates with reduction of dioxygen to water. MCOs oxidize their substrate by accepting electrons at a mononuclear copper centre and transferring them to a trinuclear copper centre which binds a dioxygen. The dioxygen, following the transfer of four electrons, is reduced to two molecules of water. These MCOs are capable of oxidizing a vast range of substrates, varying from aromatic to inorganic compounds such as metals. This subfamily of MCOs is composed of three cupredoxin domains. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 225043 | SufI | 3.35e-61 | 44 | 518 | 64 | 446 | Multicopper oxidase with three cupredoxin domains (includes cell division protein FtsP and spore coat protein CotA) [Cell cycle control, cell division, chromosome partitioning, Inorganic ion transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| 259945 | CuRO_2_Fet3p_like | 1.00e-50 | 167 | 307 | 1 | 148 | The second Cupredoxin domain of multicopper oxidase Fet3P. Fet3p catalyzes the ferroxidase reaction, which couples the oxidation of Fe(II) to Fe(III) with the four-electron reduction of molecular oxygen to water. Fet3p is a type I membrane protein with the amino-terminal oxidase domain in the extracellular space and the carboxyl terminus in the cytoplasm. The periplasmic produced Fe(III) is transferred to the permease Ftr1p for import into the cytosol. The four copper ions are inserted post-translationally and are essential for catalytic activity, thus linking copper and iron homeostasis. Like other related multicopper oxidases (MCOs), Fet3p is composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 2 of 3-domain MCOs has lost the ability to bind copper. |
| 259966 | CuRO_3_Fet3p | 1.85e-35 | 374 | 520 | 12 | 158 | The third Cupredoxin domain of multicopper oxidase Fet3p. Fet3p catalyzes the ferroxidase reaction, which couples the oxidation of Fe(II) to Fe(III) with the four-electron reduction of molecular oxygen to water. Fet3p is a type I membrane protein with the amino-terminal oxidase domain in the extracellular space and the carboxyl terminus in the cytoplasm. The periplasmic produced Fe(III) is transferred to the permease Ftr1p for import into the cytosol. The four copper ions are inserted post-translationally and are essential for catalytic activity, thus linking copper and iron homeostasis. Like other related multicopper oxidases (MCOs), Fet3p is composed of three cupredoxin domains that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 3 of 3-domain MCOs contains the Type 1 (T1) copper binding site and part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 274555 | ascorbase | 5.40e-34 | 30 | 523 | 31 | 526 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 612 | 1 | 607 | |
| 2.20e-265 | 10 | 607 | 17 | 635 | |
| 3.90e-265 | 10 | 607 | 17 | 651 | |
| 1.81e-263 | 10 | 535 | 17 | 572 | |
| 1.28e-173 | 6 | 555 | 14 | 565 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.48e-47 | 26 | 520 | 14 | 479 | Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae],1ZPU_B Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae],1ZPU_C Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae],1ZPU_D Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae],1ZPU_E Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae],1ZPU_F Crystal Structure of Fet3p, a Multicopper Oxidase that Functions in Iron Import [Saccharomyces cerevisiae] |
|
| 1.00e-40 | 61 | 531 | 77 | 500 | Chain A, Laccase [Rigidoporus microporus] |
|
| 2.16e-36 | 44 | 522 | 34 | 470 | Chain A, LACCASE 1 [Coprinopsis cinerea] |
|
| 2.18e-36 | 44 | 522 | 34 | 470 | Chain A, Laccase [Coprinopsis cinerea] |
|
| 4.82e-34 | 51 | 525 | 105 | 547 | Crystal structure of laccase from Botrytis aclada at 1.67 A resolution [Botrytis aclada],4X4K_A Structure of laccase from Botrytis aclada with full copper content [Botrytis aclada] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.78e-139 | 6 | 548 | 14 | 571 | Putative multicopper oxidase GMC1 OS=Saccharomyces cerevisiae (strain ATCC 204508 / S288c) OX=559292 GN=GMC1 PE=1 SV=1 |
|
| 4.37e-49 | 45 | 526 | 59 | 503 | Iron transport multicopper oxidase fio1 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=fio1 PE=3 SV=1 |
|
| 6.00e-49 | 44 | 540 | 54 | 521 | Iron transport multicopper oxidase fetC OS=Epichloe festucae (strain E2368) OX=696363 GN=fetC PE=2 SV=1 |
|
| 1.38e-48 | 24 | 528 | 32 | 496 | Iron transport multicopper oxidase fetC OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=fetC PE=2 SV=1 |
|
| 5.64e-48 | 38 | 538 | 49 | 516 | Iron transport multicopper oxidase FET3 OS=Candida albicans OX=5476 GN=FET3 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
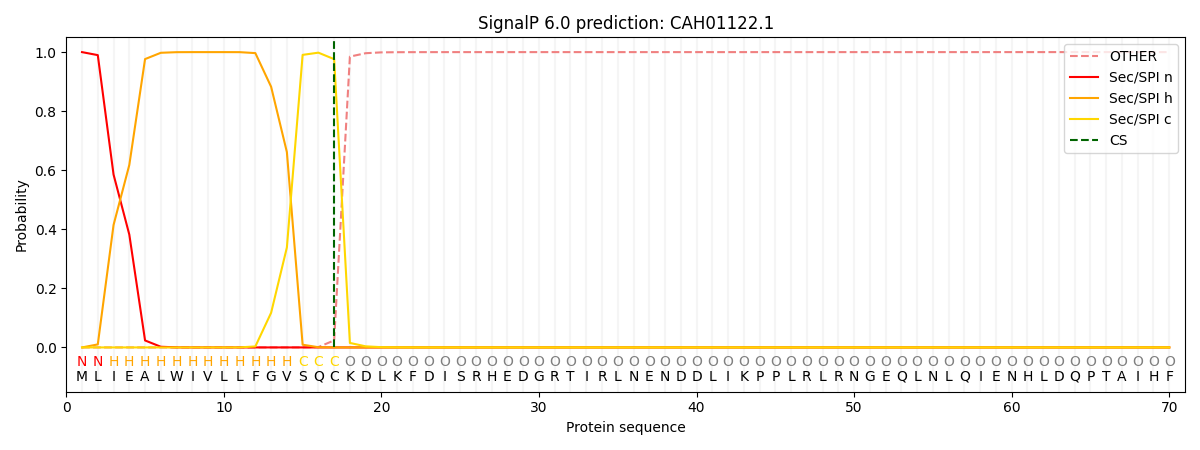
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000212 | 0.999754 | CS pos: 17-18. Pr: 0.9761 |
