You are browsing environment: FUNGIDB
CAZyme Information: An19g00100-T-p1
You are here: Home > Sequence: An19g00100-T-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
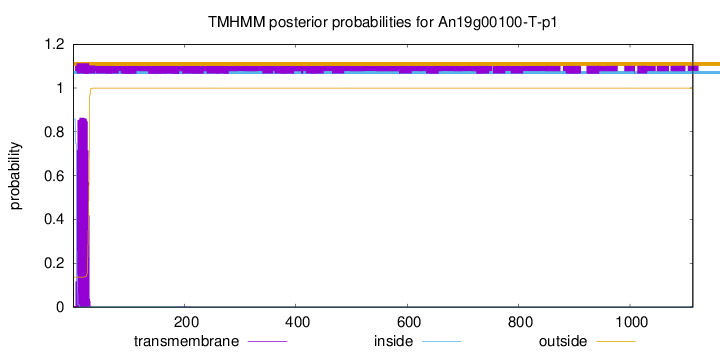
TMHMM annotations
Basic Information help
| Species | Aspergillus niger | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus niger | |||||||||||
| CAZyme ID | An19g00100-T-p1 | |||||||||||
| CAZy Family | GT62 | |||||||||||
| CAZyme Description | Putative chitinase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.14:2 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH18 | 161 | 505 | 1.8e-64 | 0.9459459459459459 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 214753 | Glyco_18 | 1.22e-76 | 162 | 502 | 1 | 334 | Glyco_18 domain. |
| 119357 | GH18_zymocin_alpha | 2.32e-74 | 164 | 499 | 3 | 342 | Zymocin, alpha subunit. Zymocin is a heterotrimeric enzyme that inhibits yeast cell cycle progression. The zymocin alpha subunit has a chitinase activity that is essential for holoenzyme action from the cell exterior while the gamma subunit contains the intracellular toxin responsible for G1 phase cell cycle arrest. The zymocin alpha and beta subunits are thought to act from the cell's exterior by docking to the cell wall-associated chitin, thus mediating gamma-toxin translocation. The alpha subunit has an eight-stranded TIM barrel fold similar to that of family 18 glycosyl hydrolases such as hevamine, chitolectin, and chitobiase. |
| 119351 | GH18_chitolectin_chitotriosidase | 1.80e-67 | 163 | 506 | 1 | 345 | This conserved domain family includes a large number of catalytically inactive chitinase-like lectins (chitolectins) including YKL-39, YKL-40 (HCGP39), YM1, oviductin, and AMCase (acidic mammalian chitinase), as well as catalytically active chitotriosidases. The conserved domain is an eight-stranded alpha/beta barrel fold belonging to the family 18 glycosyl hydrolases. The fold has a pronounced active-site cleft at the C-terminal end of the beta-barrel. The chitolectins lack a key active site glutamate (the proton donor required for hydrolytic activity) but retain highly conserved residues involved in oligosaccharide binding. Chitotriosidase is a chitinolytic enzyme expressed in maturing macrophages, which suggests that it plays a part in antimicrobial defense. Chitotriosidase hydrolyzes chitotriose, as well as colloidal chitin to yield chitobiose and is therefore considered an exochitinase. Chitotriosidase occurs in two major forms, the large form being converted to the small form by either RNA or post-translational processing. Although the small form, containing the chitinase domain alone, is sufficient for the chitinolytic activity, the additional C-terminal chitin-binding domain of the large form plays a role in processing colloidal chitin. The chitotriosidase gene is nonessential in humans, as about 35% of the population are heterozygous and 6% homozygous for an inactivated form of the gene. HCGP39 is a 39-kDa human cartilage glycoprotein thought to play a role in connective tissue remodeling and defense against pathogens. |
| 395573 | Glyco_hydro_18 | 4.02e-66 | 162 | 502 | 1 | 307 | Glycosyl hydrolases family 18. |
| 119365 | GH18_chitinase | 1.81e-52 | 163 | 502 | 1 | 322 | The GH18 (glycosyl hydrolases, family 18) type II chitinases hydrolyze chitin, an abundant polymer of N-acetylglucosamine and have been identified in bacteria, fungi, insects, plants, viruses, and protozoan parasites. The structure of this domain is an eight-stranded alpha/beta barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 1113 | 1 | 1113 | |
| 5.48e-255 | 11 | 1110 | 8 | 1107 | |
| 2.07e-241 | 28 | 1107 | 18 | 1061 | |
| 3.10e-241 | 19 | 1108 | 12 | 1038 | |
| 7.45e-236 | 28 | 868 | 21 | 830 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.34e-39 | 162 | 506 | 2 | 346 | High resoultion crystal structure of human chitinase in complex with allosamidin [Homo sapiens] |
|
| 1.37e-39 | 162 | 506 | 2 | 346 | Crystal structure of human chitinase in complex with glucoallosamidin B [Homo sapiens],1HKJ_A Crystal structure of human chitinase in complex with methylallosamidin [Homo sapiens],1HKM_A High resolution crystal structure of human chitinase in complex with demethylallosamidin [Homo sapiens] |
|
| 1.37e-39 | 162 | 506 | 2 | 346 | Crystal Structure Of Human Chitotriosidase In Complex With Chitobiose [Homo sapiens],1LG2_A Crystal Structure Of Human Chitotriosidase In Complex With Ethylene Glycol [Homo sapiens],1LQ0_A Crystal Structure Of Human Chitotriosidase At 2.2 Angstrom Resolution [Homo sapiens],6ZE8_A Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens],6ZE8_B Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens],6ZE8_C Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens],6ZE8_D Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens],6ZE8_E Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens],6ZE8_F Crystal structure of human chitotriosidase-1 (hCHIT) catalytic domain in complex with compound OATD-01 [Homo sapiens] |
|
| 1.40e-39 | 162 | 506 | 2 | 346 | Structure of human chitotriosidase [Homo sapiens] |
|
| 1.44e-39 | 162 | 506 | 8 | 363 | Crystal structure of Ostrinia furnacalis Group I chitinase catalytic domain in complex with reaction products (GlcNAc)2,3 [Ostrinia furnacalis],3WQV_A Crystal structure of Ostrinia furnacalis Group I chitinase catalytic domain in complex with a(GlcN)5 [Ostrinia furnacalis],3WQW_A Crystal structure of Ostrinia furnacalis Group I chitinase catalytic domain in complex with a(GlcN)6 [Ostrinia furnacalis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.47e-39 | 170 | 505 | 389 | 715 | Killer toxin subunits alpha/beta OS=Kluyveromyces lactis (strain ATCC 8585 / CBS 2359 / DSM 70799 / NBRC 1267 / NRRL Y-1140 / WM37) OX=284590 PE=1 SV=1 |
|
| 2.96e-38 | 155 | 506 | 16 | 367 | Chitotriosidase-1 OS=Homo sapiens OX=9606 GN=CHIT1 PE=1 SV=1 |
|
| 4.34e-37 | 166 | 508 | 27 | 371 | Chitinase-like protein 4 OS=Mus musculus OX=10090 GN=Chil4 PE=1 SV=2 |
|
| 1.24e-36 | 153 | 506 | 15 | 379 | Endochitinase OS=Manduca sexta OX=7130 PE=2 SV=1 |
|
| 6.04e-36 | 166 | 508 | 27 | 371 | Chitinase-like protein 3 OS=Mus musculus OX=10090 GN=Chil3 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
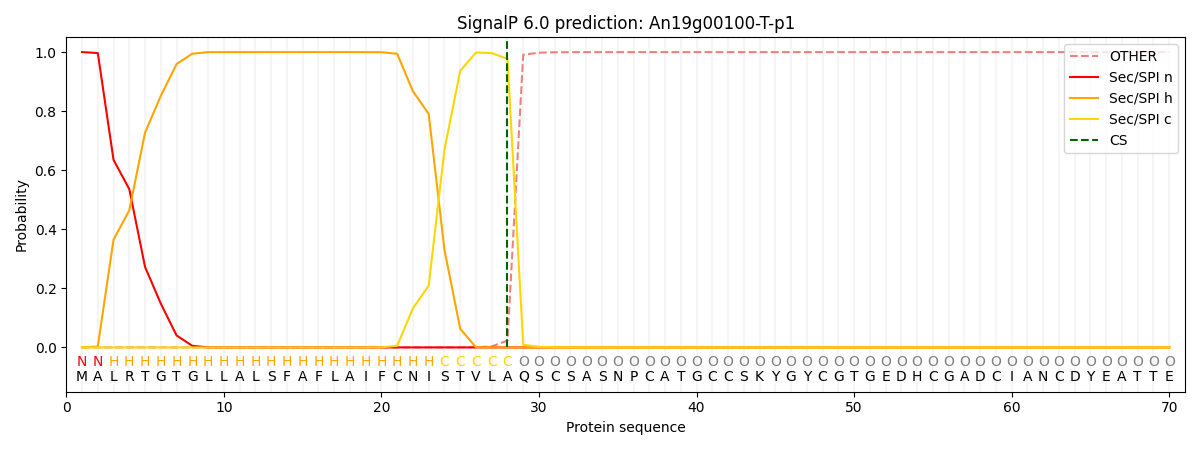
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000258 | 0.999734 | CS pos: 28-29. Pr: 0.9769 |