You are browsing environment: FUNGIDB
CAZyme Information: Afu3g00610-T-p1
You are here: Home > Sequence: Afu3g00610-T-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus fumigatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus fumigatus | |||||||||||
| CAZyme ID | Afu3g00610-T-p1 | |||||||||||
| CAZy Family | GH15 | |||||||||||
| CAZyme Description | Has domain(s) with predicted catalytic activity, glucan 1,4-alpha-glucosidase activity, hydrolase activity, hydrolyzing O-glycosyl compounds, starch binding activity and role in polysaccharide metabolic process | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.3:94 | 3.2.1.1:13 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH15 | 49 | 463 | 2.7e-74 | 0.9778393351800554 |
| CBM20 | 517 | 606 | 6.8e-21 | 0.9444444444444444 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 395586 | Glyco_hydro_15 | 6.59e-130 | 46 | 464 | 5 | 415 | Glycosyl hydrolases family 15. In higher organisms this family is represented by phosphorylase kinase subunits. |
| 99886 | CBM20_glucoamylase | 1.33e-41 | 511 | 617 | 1 | 106 | Glucoamylase (glucan1,4-alpha-glucosidase), C-terminal CBM20 (carbohydrate-binding module, family 20) domain. Glucoamylases are inverting, exo-acting starch hydrolases that hydrolyze starch and related polysaccharides by releasing the nonreducing end glucose. They are mainly active on alpha-1,4-glycosidic bonds but also have some activity towards 1,6-glycosidic bonds occurring in natural oligosaccharides. The ability of glucoamylases to cleave 1-6-glycosidic binds is called "debranching activity" and is of importance in industrial applications, where complete degradation of starch to glucose is needed. Most glucoamylases are multidomain proteins containing an N-terminal catalytic domain, a C-terminal CBM20 domain, and a highly O-glycosylated linker region that connects the two. The CBM20 domain is found in a large number of starch degrading enzymes including alpha-amylase, beta-amylase, glucoamylase, and CGTase (cyclodextrin glucanotransferase). CBM20 is also present in proteins that have a regulatory role in starch metabolism in plants (e.g. alpha-amylase) or glycogen metabolism in mammals (e.g. laforin). CBM20 folds as an antiparallel beta-barrel structure with two starch binding sites. These two sites are thought to differ functionally with site 1 acting as the initial starch recognition site and site 2 involved in the specific recognition of appropriate regions of starch. |
| 395557 | CBM_20 | 1.76e-31 | 517 | 613 | 1 | 95 | Starch binding domain. |
| 215006 | CBM_2 | 6.72e-25 | 517 | 605 | 1 | 88 | Starch binding domain. |
| 119437 | CBM20 | 9.95e-22 | 519 | 607 | 2 | 88 | The family 20 carbohydrate-binding module (CBM20), also known as the starch-binding domain, is found in a large number of starch degrading enzymes including alpha-amylase, beta-amylase, glucoamylase, and CGTase (cyclodextrin glucanotransferase). CBM20 is also present in proteins that have a regulatory role in starch metabolism in plants (e.g. alpha-amylase) or glycogen metabolism in mammals (e.g. laforin). CBM20 folds as an antiparallel beta-barrel structure with two starch binding sites. These two sites are thought to differ functionally with site 1 acting as the initial starch recognition site and site 2 involved in the specific recognition of appropriate regions of starch. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 4.46e-271 | 39 | 619 | 36 | 616 | |
| 2.01e-215 | 21 | 618 | 19 | 616 | |
| 8.11e-215 | 24 | 618 | 22 | 616 | |
| 3.44e-214 | 34 | 619 | 70 | 679 | |
| 5.96e-214 | 40 | 619 | 63 | 654 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.68e-272 | 39 | 618 | 14 | 593 | Crystal structure of Penicillium oxalicum Glucoamylase [Penicillium oxalicum 114-2] |
|
| 5.43e-212 | 44 | 619 | 13 | 599 | Chain A, GLUCOAMYLASE [Trichoderma reesei],2VN7_A Chain A, GLUCOAMYLASE [Trichoderma reesei] |
|
| 4.41e-205 | 34 | 607 | 4 | 604 | Structure of the catalytic domain of Aspergillus niger Glucoamylase [Aspergillus niger] |
|
| 4.03e-184 | 34 | 500 | 4 | 461 | Catalytic domain of glucoamylase from aspergillus niger complexed with tris and glycerol [Aspergillus niger] |
|
| 1.43e-182 | 34 | 617 | 32 | 606 | Chain A, Glucoamylase P [Amorphotheca resinae],6FHW_B Chain B, Glucoamylase P [Amorphotheca resinae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.11e-204 | 34 | 607 | 28 | 628 | Glucoamylase OS=Aspergillus awamori OX=105351 GN=GLAA PE=1 SV=1 |
|
| 5.11e-204 | 34 | 607 | 28 | 628 | Glucoamylase OS=Aspergillus niger OX=5061 GN=GLAA PE=1 SV=1 |
|
| 2.57e-203 | 34 | 618 | 38 | 626 | Glucoamylase OS=Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) OX=367110 GN=gla-1 PE=1 SV=3 |
|
| 2.60e-201 | 34 | 607 | 28 | 627 | Glucoamylase OS=Aspergillus usamii OX=186680 GN=glaA PE=3 SV=1 |
|
| 1.48e-200 | 34 | 607 | 28 | 627 | Glucoamylase I OS=Aspergillus kawachii OX=1069201 GN=gaI PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
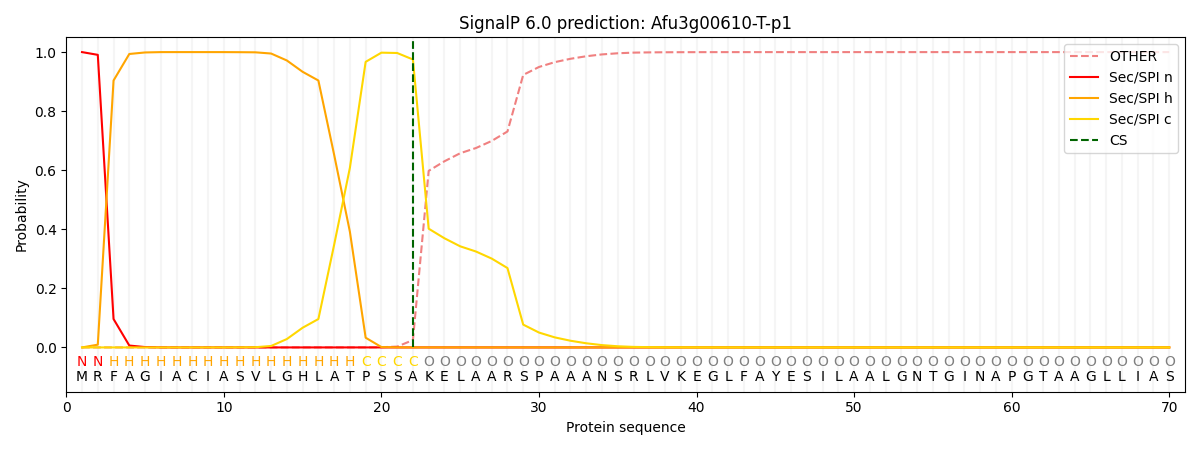
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000394 | 0.999591 | CS pos: 22-23. Pr: 0.9750 |
