You are browsing environment: FUNGIDB
CAZyme Information: ATEG_08627-t26_1-p1
You are here: Home > Sequence: ATEG_08627-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus terreus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus terreus | |||||||||||
| CAZyme ID | ATEG_08627-t26_1-p1 | |||||||||||
| CAZy Family | GT15 | |||||||||||
| CAZyme Description | conserved hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE2 | 179 | 372 | 6.7e-20 | 0.861244019138756 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 238869 | Endoglucanase_E_like | 1.25e-50 | 165 | 375 | 1 | 169 | Endoglucanase E-like members of the SGNH hydrolase family; Endoglucanase E catalyzes the endohydrolysis of 1,4-beta-glucosidic linkages in cellulose, lichenin and cereal beta-D-glucans. |
| 238141 | SGNH_hydrolase | 2.41e-12 | 233 | 372 | 44 | 186 | SGNH_hydrolase, or GDSL_hydrolase, is a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxlic acid. |
| 404371 | Lipase_GDSL_2 | 3.99e-09 | 168 | 363 | 1 | 174 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| 395531 | Lipase_GDSL | 0.007 | 245 | 370 | 68 | 224 | GDSL-like Lipase/Acylhydrolase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 5.11e-179 | 1 | 401 | 1 | 407 | |
| 2.26e-169 | 18 | 401 | 17 | 402 | |
| 3.14e-166 | 6 | 401 | 7 | 409 | |
| 4.29e-159 | 1 | 401 | 1 | 379 | |
| 5.50e-158 | 18 | 401 | 17 | 392 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
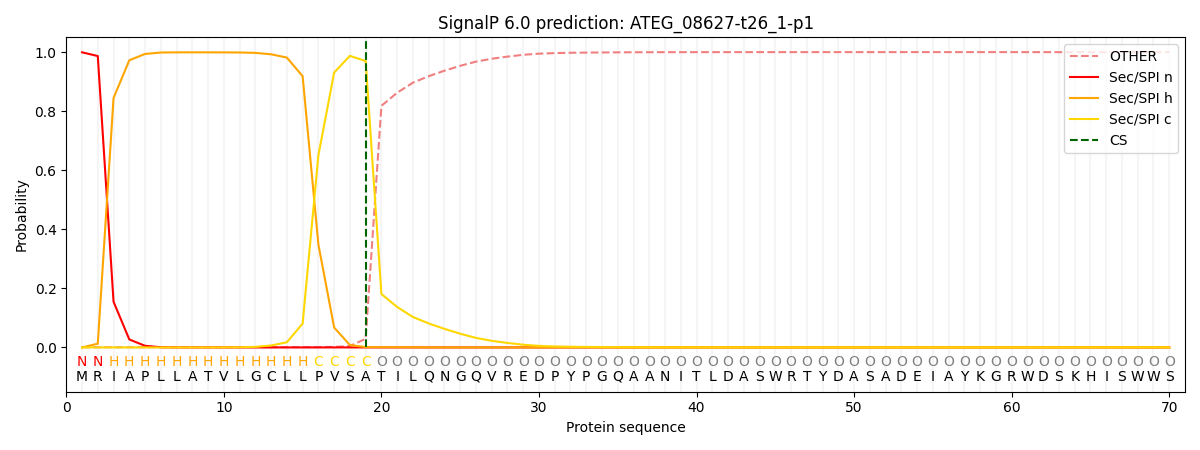
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.002300 | 0.997668 | CS pos: 19-20. Pr: 0.9697 |
