You are browsing environment: FUNGIDB
CAZyme Information: ATCC64974_102960-t41_1-p1
You are here: Home > Sequence: ATCC64974_102960-t41_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus niger | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus niger | |||||||||||
| CAZyme ID | ATCC64974_102960-t41_1-p1 | |||||||||||
| CAZy Family | AA1 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.109:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH114 | 34 | 224 | 2.7e-72 | 0.9947368421052631 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 397552 | Glyco_hydro_114 | 5.24e-98 | 33 | 263 | 1 | 220 | Glycoside-hydrolase family GH114. This family is recognized as a glycosyl-hydrolase family, number 114. It is endo-alpha-1,4-polygalactosaminidase, a rare enzyme. It is proposed to be TIM-barrel, the most common structure amongst the catalytic domains of glycosyl-hydrolases. |
| 226386 | COG3868 | 8.20e-28 | 13 | 224 | 49 | 266 | Predicted glycosyl hydrolase, GH114 family [Carbohydrate transport and metabolism]. |
| 225219 | COG2342 | 4.78e-07 | 61 | 258 | 60 | 281 | Endo alpha-1,4 polygalactosaminidase, GH114 family (was erroneously annotated as Cys-tRNA synthetase) [Carbohydrate transport and metabolism]. |
| 273582 | TIGR01370 | 3.56e-05 | 61 | 233 | 84 | 282 | extracellular protein. Original assignment of this protein family as cysteinyl-tRNA synthetase is controversial, supported by but challenged by and by subsequent discovery of the actual mechanism for synthesizing Cys-tRNA in species where a direct Cys--tRNA ligase was not found. Lingering legacy annotations of members of this family probably should be removed. Evidence against the role includes a signal peptide. This family as been renamed "extracellular protein" to facilitate correction. Members of this family occur in Deinococcus radiodurans (bacterial) and Methanococcus jannaschii (archaeal). A number of homologous but more distantly related proteins are annotated as alpha-1,4 polygalactosaminidases. The function remains unknown. [Unknown function, General] |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 6.24e-201 | 1 | 270 | 1 | 270 | |
| 3.52e-182 | 1 | 270 | 1 | 270 | |
| 5.67e-182 | 1 | 270 | 14 | 283 | |
| 6.02e-105 | 24 | 269 | 71 | 316 | |
| 2.48e-97 | 3 | 269 | 3 | 276 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.55e-93 | 26 | 269 | 34 | 277 | Crystal Structure of Aspergillus fumigatus Ega3 [Aspergillus fumigatus Af293] |
|
| 4.44e-93 | 26 | 269 | 51 | 294 | Crystal Structure of Aspergillus fumigatus Ega3 complex with galactosamine [Aspergillus fumigatus Af293] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
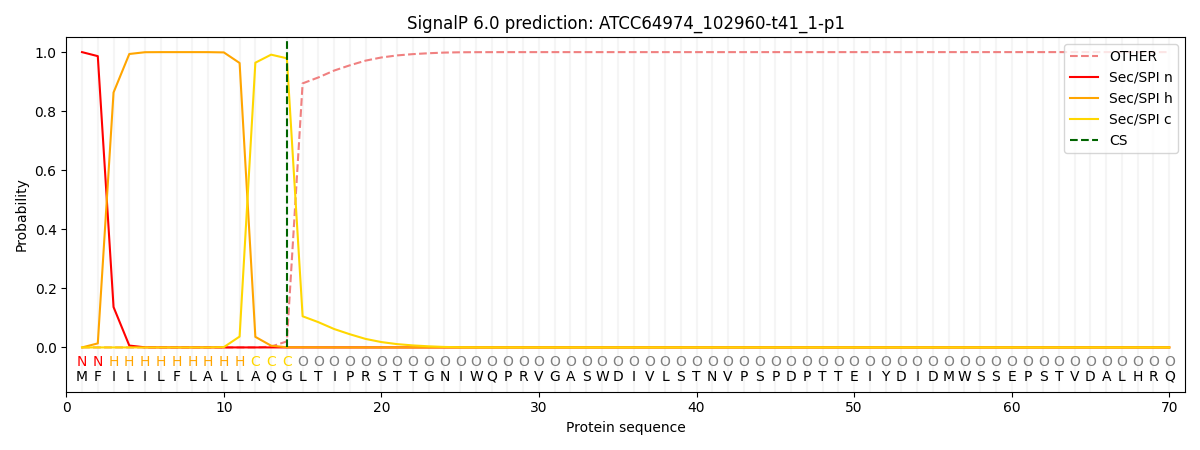
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000238 | 0.999709 | CS pos: 14-15. Pr: 0.9790 |
