You are browsing environment: FUNGIDB
CAZyme Information: ASPWEDRAFT_34175-t33_1-p1
You are here: Home > Sequence: ASPWEDRAFT_34175-t33_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus wentii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus wentii | |||||||||||
| CAZyme ID | ASPWEDRAFT_34175-t33_1-p1 | |||||||||||
| CAZy Family | GH32 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.1:16 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 58 | 362 | 3.6e-139 | 0.9967320261437909 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 200458 | AmyAc_euk_AmyA | 0.0 | 20 | 392 | 1 | 373 | Alpha amylase catalytic domain found in eukaryotic Alpha-amylases (also called 1,4-alpha-D-glucan-4-glucanohydrolase). AmyA (EC 3.2.1.1) catalyzes the hydrolysis of alpha-(1,4) glycosidic linkages of glycogen, starch, related polysaccharides, and some oligosaccharides. This group includes eukaryotic alpha-amylases including proteins from fungi, sponges, and protozoans. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| 395077 | Alpha-amylase | 7.37e-90 | 59 | 360 | 1 | 334 | Alpha amylase, catalytic domain. Alpha amylase is classified as family 13 of the glycosyl hydrolases. The structure is an 8 stranded alpha/beta barrel containing the active site, interrupted by a ~70 a.a. calcium-binding domain protruding between beta strand 3 and alpha helix 3, and a carboxyl-terminal Greek key beta-barrel domain. |
| 200478 | AmyAc_bac_CMD_like_2 | 7.51e-81 | 26 | 389 | 1 | 342 | Alpha amylase catalytic domain found in bacterial cyclomaltodextrinases and related proteins. Cyclomaltodextrinase (CDase; EC3.2.1.54), neopullulanase (NPase; EC 3.2.1.135), and maltogenic amylase (MA; EC 3.2.1.133) catalyze the hydrolysis of alpha-(1,4) glycosidic linkages on a number of substrates including cyclomaltodextrins (CDs), pullulan, and starch. These enzymes hydrolyze CDs and starch to maltose and pullulan to panose by cleavage of alpha-1,4 glycosidic bonds whereas alpha-amylases essentially lack activity on CDs and pullulan. They also catalyze transglycosylation of oligosaccharides to the C3-, C4- or C6-hydroxyl groups of various acceptor sugar molecules. Since these proteins are nearly indistinguishable from each other, they are referred to as cyclomaltodextrinases (CMDs). This group of CMDs is bacterial. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| 200459 | AmyAc_AmyMalt_CGTase_like | 1.70e-71 | 26 | 388 | 3 | 387 | Alpha amylase catalytic domain found in maltogenic amylases, cyclodextrin glycosyltransferase, and related proteins. Enzymes such as amylases, cyclomaltodextrinase (CDase), and cyclodextrin glycosyltransferase (CGTase) degrade starch to smaller oligosaccharides by hydrolyzing the alpha-D-(1,4) linkages between glucose residues. In the case of CGTases, an additional cyclization reaction is catalyzed yielding mixtures of cyclic oligosaccharides which are referred to as alpha-, beta-, or gamma-cyclodextrins (CDs), consisting of six, seven, or eight glucose residues, respectively. CGTases are characterized depending on the major product of the cyclization reaction. Besides having similar catalytic site residues, amylases and CGTases contain carbohydrate binding domains that are distant from the active site and are implicated in attaching the enzyme to raw starch granules and in guiding the amylose chain into the active site. The maltogenic alpha-amylase from Bacillus is a five-domain structure, unlike most alpha-amylases, but similar to that of cyclodextrin glycosyltransferase. In addition to the A, B, and C domains, they have a domain D and a starch-binding domain E. Maltogenic amylase is an endo-acting amylase that has activity on cyclodextrins, terminally modified linear maltodextrins, and amylose. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| 200479 | AmyAc_bac_CMD_like_3 | 4.02e-50 | 30 | 364 | 6 | 368 | Alpha amylase catalytic domain found in bacterial cyclomaltodextrinases and related proteins. Cyclomaltodextrinase (CDase; EC3.2.1.54), neopullulanase (NPase; EC 3.2.1.135), and maltogenic amylase (MA; EC 3.2.1.133) catalyze the hydrolysis of alpha-(1,4) glycosidic linkages on a number of substrates including cyclomaltodextrins (CDs), pullulan, and starch. These enzymes hydrolyze CDs and starch to maltose and pullulan to panose by cleavage of alpha-1,4 glycosidic bonds whereas alpha-amylases essentially lack activity on CDs and pullulan. They also catalyze transglycosylation of oligosaccharides to the C3-, C4- or C6-hydroxyl groups of various acceptor sugar molecules. Since these proteins are nearly indistinguishable from each other, they are referred to as cyclomaltodextrinases (CMDs). This group of CMDs is bacterial. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.61e-288 | 5 | 495 | 5 | 494 | |
| 8.76e-288 | 1 | 495 | 2 | 497 | |
| 3.43e-287 | 1 | 494 | 2 | 496 | |
| 4.48e-280 | 8 | 495 | 5 | 493 | |
| 1.28e-279 | 8 | 495 | 5 | 493 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.44e-280 | 1 | 495 | 2 | 497 | Taka-amylase [Aspergillus oryzae],6YQ7_B Taka-amylase [Aspergillus oryzae],6YQ9_AAA Chain AAA, Alpha-amylase [Aspergillus oryzae],6YQ9_BBB Chain BBB, Alpha-amylase [Aspergillus oryzae],6YQA_AAA Chain AAA, Alpha-amylase [Aspergillus oryzae],6YQA_BBB Chain BBB, Alpha-amylase [Aspergillus oryzae],6YQB_AAA Chain AAA, Alpha-amylase [Aspergillus oryzae],6YQB_BBB Chain BBB, Alpha-amylase [Aspergillus oryzae],6YQC_AAA Chain AAA, Alpha-amylase [Aspergillus oryzae],6YQC_BBB Chain BBB, Alpha-amylase [Aspergillus oryzae] |
|
| 1.44e-280 | 1 | 495 | 2 | 497 | Chain A, Alpha-amylase [Aspergillus oryzae],6XSJ_B Chain B, Alpha-amylase [Aspergillus oryzae],6XSV_A Chain A, Alpha-amylase [Aspergillus oryzae] |
|
| 1.59e-276 | 20 | 495 | 1 | 476 | Chain A, Alpha-amylase [Aspergillus oryzae] |
|
| 1.71e-276 | 20 | 495 | 1 | 476 | Orthorhombic crystal structure (space group P21212) of Aspergillus niger alpha-amylase at 1.6 A resolution [Aspergillus oryzae],2GVY_A Monoclinic crystal form of Aspergillus niger alpha-amylase in complex with maltose at 1.8 A resolution [Aspergillus oryzae],2GVY_B Monoclinic crystal form of Aspergillus niger alpha-amylase in complex with maltose at 1.8 A resolution [Aspergillus oryzae],3KWX_A Chemically modified Taka alpha-amylase [Aspergillus oryzae],3VX0_A Crystal Structure of alpha-amylase from Aspergillus oryzae [Aspergillus oryzae RIB40],3VX1_A Crystal Structure of alpha-Amylase from Aspergillus oryzae [Aspergillus oryzae RIB40],6TAA_A Structure And Molecular Model Refinement Of Aspergillus Oryzae (Taka) Alpha-Amylase: An Application Of The Simulated-Annealing Method [Aspergillus oryzae],7TAA_A Family 13 Alpha Amylase In Complex With Acarbose [Aspergillus oryzae] |
|
| 1.99e-275 | 20 | 495 | 1 | 475 | Structure And Possible Catalytic Residues Of Taka-amylase A [Aspergillus oryzae],2TAA_B Structure And Possible Catalytic Residues Of Taka-amylase A [Aspergillus oryzae],2TAA_C Structure And Possible Catalytic Residues Of Taka-amylase A [Aspergillus oryzae] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.56e-288 | 1 | 495 | 2 | 497 | Alpha-amylase B OS=Aspergillus awamori OX=105351 GN=amyB PE=3 SV=1 |
|
| 6.09e-288 | 1 | 494 | 2 | 496 | Alpha-amylase A OS=Aspergillus awamori OX=105351 GN=amyA PE=3 SV=1 |
|
| 7.39e-280 | 1 | 495 | 2 | 497 | Alpha-amylase A type-1/2 OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=amy1 PE=1 SV=1 |
|
| 7.39e-280 | 1 | 495 | 2 | 497 | Alpha-amylase A type-3 OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=amy3 PE=3 SV=1 |
|
| 1.73e-278 | 1 | 495 | 2 | 497 | Alpha-amylase OS=Aspergillus usamii OX=186680 GN=amy PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
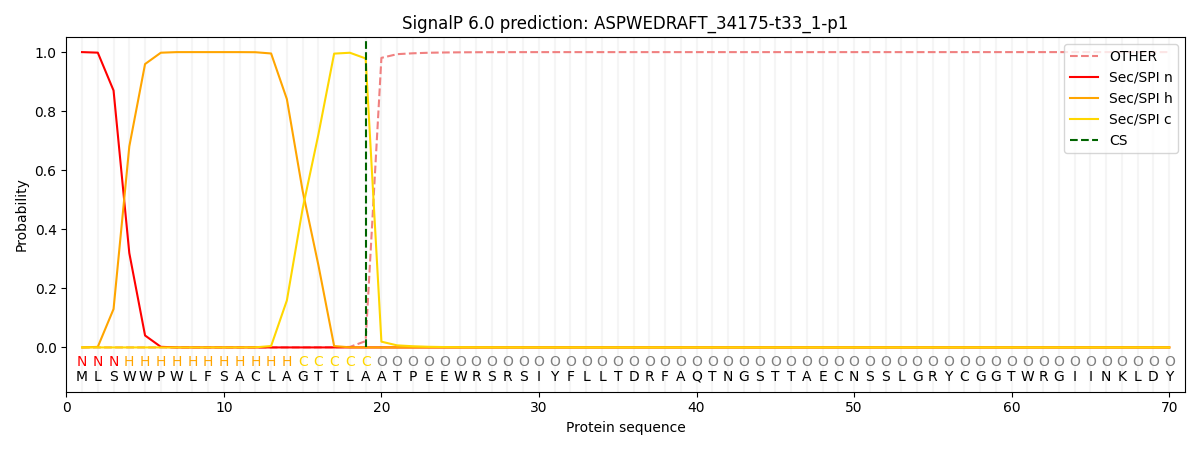
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000251 | 0.999706 | CS pos: 19-20. Pr: 0.9785 |
