You are browsing environment: FUNGIDB
CAZyme Information: APA10045.1
You are here: Home > Sequence: APA10045.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Sclerotinia sclerotiorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Leotiomycetes; ; Sclerotiniaceae; Sclerotinia; Sclerotinia sclerotiorum | |||||||||||
| CAZyme ID | APA10045.1 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM24 | 334 | 409 | 1.2e-27 | 0.9868421052631579 |
| CBM24 | 428 | 502 | 9.5e-17 | 0.9473684210526315 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 397634 | Glyco_hydro_71 | 7.92e-75 | 5 | 295 | 40 | 370 | Glycosyl hydrolase family 71. Family of alpha-1,3-glucanases. |
| 211418 | GH71 | 7.38e-52 | 1 | 165 | 43 | 228 | Glycoside hydrolase family 71. This family of glycoside hydrolases 71 (following the CAZY nomenclature) function as alpha-1,3-glucanases (mutanases, EC 3.2.1.59). They appear to have an endo-hydrolytic mode of enzymatic activity and bacterial members are investigated as candidates for the development of dental caries treatments.The member from fission yeast, endo-alpha-1,3-glucanase Agn1p, plays a vital role in daughter cell separation, while Agn2p has been associated with endolysis of the ascus wall. |
| 173790 | Peptidases_S8_PCSK9_ProteinaseK_like | 9.70e-25 | 974 | 1247 | 14 | 253 | Peptidase S8 family domain in ProteinaseK-like proteins. The peptidase S8 or Subtilase clan of proteases have a Asp/His/Ser catalytic triad that is not homologous to trypsin. This CD contains several members of this clan including: PCSK9 (Proprotein convertase subtilisin/kexin type 9), Proteinase_K, Proteinase_T, and other subtilisin-like serine proteases. PCSK9 posttranslationally regulates hepatic low-density lipoprotein receptors (LDLRs) by binding to LDLRs on the cell surface, leading to their degradation. The binding site of PCSK9 has been localized to the epidermal growth factor-like repeat A (EGF-A) domain of the LDLR. Characterized Proteinases K are secreted endopeptidases with a high degree of sequence conservation. Proteinases K are not substrate-specific and function in a wide variety of species in different pathways. It can hydrolyze keratin and other proteins with subtilisin-like specificity. The number of calcium-binding motifs found in these differ. Proteinase T is a novel proteinase from the fungus Tritirachium album Limber. The amino acid sequence of proteinase T as deduced from the nucleotide sequence is about 56% identical to that of proteinase K. |
| 173787 | Peptidases_S8_S53 | 1.19e-19 | 989 | 1244 | 3 | 239 | Peptidase domain in the S8 and S53 families. Members of the peptidases S8 (subtilisin and kexin) and S53 (sedolisin) family include endopeptidases and exopeptidases. The S8 family has an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. Serine acts as a nucleophile, aspartate as an electrophile, and histidine as a base. The S53 family contains a catalytic triad Glu/Asp/Ser with an additional acidic residue Asp in the oxyanion hole, similar to that of subtilisin. The serine residue here is the nucleophilic equivalent of the serine residue in the S8 family, while glutamic acid has the same role here as the histidine base. However, the aspartic acid residue that acts as an electrophile is quite different. In S53, it follows glutamic acid, while in S8 it precedes histidine. The stability of these enzymes may be enhanced by calcium; some members have been shown to bind up to 4 ions via binding sites with different affinity. There is a great diversity in the characteristics of their members: some contain disulfide bonds, some are intracellular while others are extracellular, some function at extreme temperatures, and others at high or low pH values. |
| 211414 | GH99_GH71_like | 1.75e-15 | 14 | 169 | 50 | 242 | Glycoside hydrolase families 71, 99, and related domains. This superfamily of glycoside hydrolases contains families GH71 and GH99 (following the CAZY nomenclature), as well as other members with undefined function and specificity. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 1421 | 1 | 1421 | |
| 2.22e-88 | 1 | 707 | 216 | 937 | |
| 1.10e-87 | 1 | 389 | 32 | 475 | |
| 1.24e-87 | 1 | 389 | 46 | 501 | |
| 2.23e-87 | 1 | 725 | 1 | 744 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.59e-07 | 1030 | 1217 | 60 | 235 | Structure of Patellamide maturation protease PatA [Prochloron didemni] |
|
| 1.60e-07 | 1030 | 1217 | 69 | 244 | Structure of self-cleaved protease domain of PatA [Prochloron didemni] |
|
| 2.12e-07 | 1031 | 1223 | 64 | 244 | Structure of Prenylagaramide maturation protease PagA [Planktothrix agardhii NIES-596],4H6W_B Structure of Prenylagaramide maturation protease PagA [Planktothrix agardhii NIES-596] |
|
| 3.15e-07 | 1030 | 1217 | 50 | 225 | Structure of S218A mutant of the protease domain of PatA [Prochloron didemni] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.92e-12 | 5 | 320 | 78 | 432 | Mutanase Pc12g07500 OS=Penicillium rubens (strain ATCC 28089 / DSM 1075 / NRRL 1951 / Wisconsin 54-1255) OX=500485 GN=PCH_Pc12g07500 PE=1 SV=1 |
|
| 2.88e-11 | 976 | 1223 | 140 | 363 | Subtilisin-like protease CPC735_066880 OS=Coccidioides posadasii (strain C735) OX=222929 GN=CPC735_066880 PE=3 SV=1 |
|
| 1.19e-10 | 949 | 1222 | 118 | 363 | Subtilisin-like protease CPC735_015300 OS=Coccidioides posadasii (strain C735) OX=222929 GN=CPC735_015300 PE=3 SV=1 |
|
| 1.20e-10 | 975 | 1222 | 140 | 364 | Subtilisin-like protease 1 (Fragment) OS=Trichophyton verrucosum OX=63417 GN=SUB1 PE=3 SV=1 |
|
| 1.20e-10 | 975 | 1222 | 140 | 364 | Subtilisin-like protease 1 (Fragment) OS=Arthroderma benhamiae OX=63400 GN=SUB1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
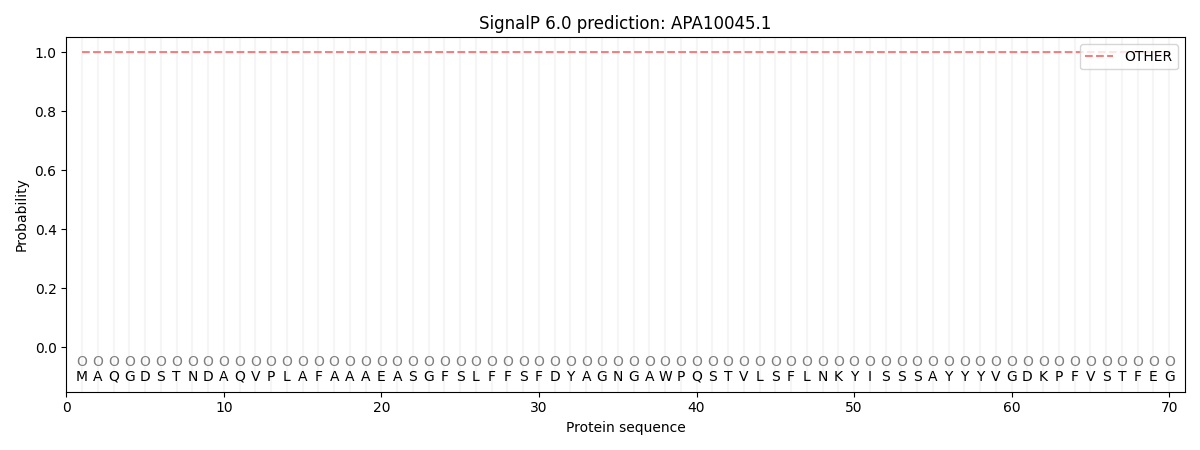
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 1.000065 | 0.000002 |
