You are browsing environment: FUNGIDB
CAZyme Information: APA09402.1
You are here: Home > Sequence: APA09402.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Sclerotinia sclerotiorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Leotiomycetes; ; Sclerotiniaceae; Sclerotinia; Sclerotinia sclerotiorum | |||||||||||
| CAZyme ID | APA09402.1 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 68 | 273 | 5e-75 | 0.43231441048034935 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 396238 | FAD_binding_4 | 2.83e-29 | 77 | 215 | 1 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 223354 | GlcD | 8.65e-27 | 74 | 268 | 29 | 228 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 211316 | ChtBD1_1 | 1.94e-18 | 562 | 602 | 1 | 43 | Hevein or type 1 chitin binding domain; filamentous ascomycete subfamily. Hevein or type 1 chitin binding domain (ChtBD1), a lectin domain found in proteins from plants and fungi that bind N-acetylglucosamine, plant endochitinases, wound-induced proteins such as hevein, a major IgE-binding allergen in natural rubber latex, and the alpha subunit of Kluyveromyces lactis killer toxin. This domain is involved in the recognition and/or binding of chitin subunits; it typically occurs N-terminal to glycosyl hydrolase domains in chitinases, together with other carbohydrate-binding domains, or by itself in tandem-repeat arrangements. |
| 395135 | Chitin_bind_1 | 2.67e-11 | 562 | 597 | 1 | 36 | Chitin recognition protein. |
| 211311 | ChtBD1 | 2.26e-09 | 562 | 598 | 1 | 37 | Hevein or type 1 chitin binding domain. Hevein or type 1 chitin binding domain (ChtBD1), a lectin domain found in proteins from plants and fungi that bind N-acetylglucosamine, plant endochitinases, wound-induced proteins such as hevein, a major IgE-binding allergen in natural rubber latex, and the alpha subunit of Kluyveromyces lactis killer toxin. This domain is involved in the recognition and/or binding of chitin subunits; it typically occurs N-terminal to glycosyl hydrolase domains in chitinases, together with other carbohydrate-binding domains, or by itself in tandem-repeat arrangements. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 603 | 1 | 603 | |
| 0.0 | 1 | 603 | 1 | 610 | |
| 0.0 | 1 | 603 | 1 | 610 | |
| 2.54e-246 | 8 | 527 | 7 | 522 | |
| 1.28e-170 | 15 | 519 | 7 | 511 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3.49e-98 | 18 | 522 | 6 | 496 | Xylooligosaccharide oxidase from Myceliophthora thermophila C1 [Thermothelomyces thermophilus ATCC 42464],5L6F_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylobiose [Thermothelomyces thermophilus ATCC 42464],5L6G_A Xylooligosaccharide oxidase from Myceliophthora thermophila C1 in complex with Xylose [Thermothelomyces thermophilus ATCC 42464] |
|
| 3.32e-87 | 18 | 521 | 9 | 504 | Chain A, FAD-binding PCMH-type domain-containing protein [Fusarium graminearum PH-1] |
|
| 9.65e-78 | 36 | 518 | 20 | 488 | Chain AAA, Chitooligosaccharide oxidase [Fusarium graminearum PH-1] |
|
| 4.32e-71 | 42 | 518 | 2 | 469 | Crystal structure of carbohydrate oxidase from Microdochium nivale [Microdochium nivale],3RJA_A Crystal structure of carbohydrate oxidase from Microdochium nivale in complex with substrate analogue [Microdochium nivale] |
|
| 1.28e-70 | 37 | 518 | 3 | 476 | The crystal structure of an Acremonium strictum glucooligosaccharide oxidase reveals a novel flavinylation [Sarocladium strictum],2AXR_A Crystal structure of glucooligosaccharide oxidase from Acremonium strictum: a novel flavinylation of 6-S-cysteinyl, 8alpha-N1-histidyl FAD [Sarocladium strictum] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.80e-97 | 18 | 522 | 6 | 496 | Xylooligosaccharide oxidase OS=Myceliophthora thermophila (strain ATCC 42464 / BCRC 31852 / DSM 1799) OX=573729 GN=xylO PE=1 SV=1 |
|
| 4.96e-77 | 36 | 518 | 20 | 488 | Chitooligosaccharide oxidase OS=Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) OX=229533 GN=chitO PE=1 SV=1 |
|
| 3.86e-70 | 42 | 518 | 24 | 491 | Carbohydrate oxidase OS=Microdochium nivale OX=5520 GN=MnCO PE=1 SV=2 |
|
| 1.16e-69 | 39 | 518 | 24 | 495 | Glucooligosaccharide oxidase OS=Sarocladium strictum OX=5046 GN=gluO PE=1 SV=1 |
|
| 3.26e-65 | 46 | 521 | 32 | 492 | FAD-linked oxidoreductase subF OS=Metarhizium robertsii (strain ARSEF 23 / ATCC MYA-3075) OX=655844 GN=subF PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
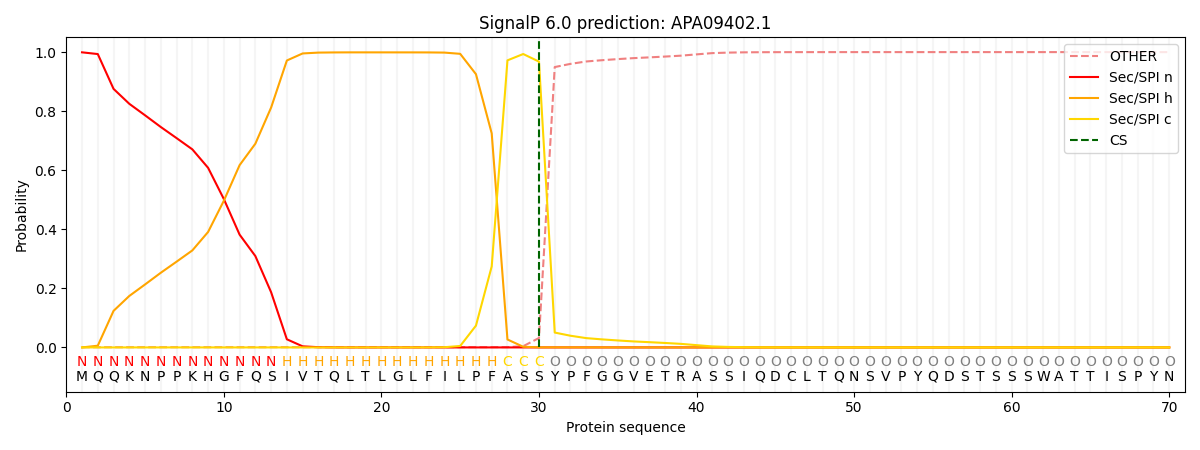
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.001697 | 0.998272 | CS pos: 30-31. Pr: 0.9678 |
