You are browsing environment: FUNGIDB
CAZyme Information: APA07533.1
You are here: Home > Sequence: APA07533.1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Sclerotinia sclerotiorum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Leotiomycetes; ; Sclerotiniaceae; Sclerotinia; Sclerotinia sclerotiorum | |||||||||||
| CAZyme ID | APA07533.1 | |||||||||||
| CAZy Family | CE5 | |||||||||||
| CAZyme Description | unspecified product | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 1.10.3.2:4 | 1.10.3.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 55 | 367 | 1e-135 | 0.9935897435897436 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 274555 | ascorbase | 4.40e-80 | 67 | 542 | 1 | 523 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
| 259923 | CuRO_1_MaLCC_like | 1.11e-75 | 65 | 186 | 1 | 122 | The first cupredoxin domain of the fungal laccases similar to Ma-LCC from Melanocarpus albomyces. The subfamily of fungal laccases includes Ma-LCC and similar proteins. Ma-LCC is a multicopper oxidase (MCO) from Melanocarpus albomyces. Its crystal structure contains all four coppers at the mono- and trinuclear copper centers. Laccase is a blue multi-copper enzyme that catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism in fungi and plants. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 274556 | laccase | 1.50e-75 | 67 | 541 | 3 | 518 | laccase, plant. Members of this protein family include the copper-containing enzyme laccase (EC 1.10.3.2), often several from a single plant species, and additional, uncharacterized, closely related plant proteins termed laccase-like multicopper oxidases. This protein family shows considerable sequence similarity to the L-ascorbate oxidase (EC 1.10.3.3) family. Laccases are enzymes of rather broad specificity, and classification of all proteins scoring about the trusted cutoff of this model as laccases may be appropriate. |
| 177843 | PLN02191 | 9.33e-75 | 60 | 547 | 14 | 551 | L-ascorbate oxidase |
| 215324 | PLN02604 | 3.66e-69 | 67 | 542 | 24 | 546 | oxidoreductase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 580 | 1 | 580 | |
| 0.0 | 1 | 580 | 1 | 580 | |
| 0.0 | 1 | 580 | 1 | 580 | |
| 0.0 | 1 | 580 | 1 | 581 | |
| 0.0 | 1 | 580 | 1 | 581 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 1 | 580 | 1 | 580 | Structure of the L499M mutant of the laccase from B.aclada [Botrytis aclada] |
|
| 0.0 | 1 | 580 | 1 | 580 | Crystal structure of laccase from Botrytis aclada at 1.67 A resolution [Botrytis aclada],4X4K_A Structure of laccase from Botrytis aclada with full copper content [Botrytis aclada] |
|
| 1.32e-154 | 41 | 567 | 25 | 556 | Crystal structure of the H253D mutant of McoG from Aspergillus niger [Aspergillus niger] |
|
| 8.57e-154 | 48 | 567 | 4 | 528 | Crystal structure of a laccase-like multicopper oxidase McoG from from Aspergillus niger [Aspergillus niger] |
|
| 8.85e-154 | 48 | 567 | 5 | 529 | Crystal structure of a laccase-like multicopper oxidase McoG from Aspergillus niger bound to zinc [Aspergillus niger] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 0.0 | 1 | 580 | 1 | 581 | Laccase-2 OS=Botryotinia fuckeliana OX=40559 GN=lcc2 PE=2 SV=1 |
|
| 6.34e-223 | 10 | 580 | 15 | 561 | Laccase-1 OS=Botryotinia fuckeliana OX=40559 GN=lcc1 PE=2 SV=3 |
|
| 6.03e-198 | 38 | 580 | 44 | 590 | Oxidoreductase OpS5 OS=Beauveria bassiana (strain ARSEF 2860) OX=655819 GN=OpS5 PE=1 SV=1 |
|
| 3.87e-143 | 36 | 580 | 26 | 605 | Dihydrogeodin oxidase OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=gedJ PE=1 SV=1 |
|
| 2.01e-139 | 31 | 580 | 29 | 591 | Laccase OS=Cryphonectria parasitica OX=5116 GN=LAC-1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
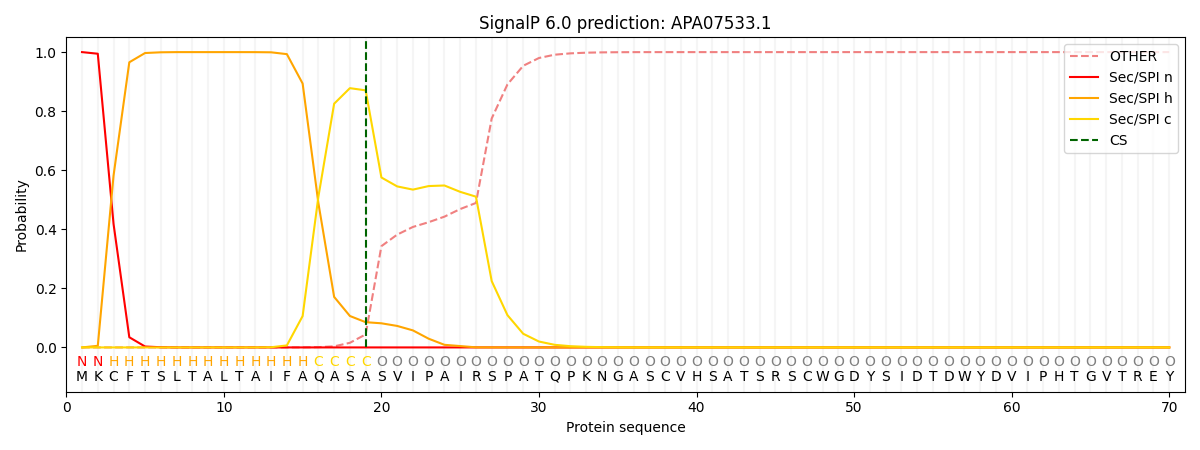
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000293 | 0.999674 | CS pos: 19-20. Pr: 0.8704 |
