You are browsing environment: FUNGIDB
CAZyme Information: AKAW_10784-t41_1-p1
You are here: Home > Sequence: AKAW_10784-t41_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus luchuensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus luchuensis | |||||||||||
| CAZyme ID | AKAW_10784-t41_1-p1 | |||||||||||
| CAZy Family | GT35 | |||||||||||
| CAZyme Description | beta-1,6-glucanase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 3.2.1.75:6 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH30 | 70 | 494 | 4.4e-124 | 0.9832134292565947 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 227807 | XynC | 3.60e-38 | 49 | 494 | 16 | 427 | O-Glycosyl hydrolase [Cell wall/membrane/envelope biogenesis]. |
| 307945 | Glyco_hydro_30 | 4.23e-31 | 82 | 424 | 1 | 348 | Glycosyl hydrolase family 30 TIM-barrel domain. |
| 407314 | Glyco_hydro_30C | 1.65e-06 | 438 | 493 | 8 | 63 | Glycosyl hydrolase family 30 beta sandwich domain. |
| 396577 | Glyco_hydro_59 | 3.55e-05 | 107 | 421 | 26 | 292 | Glycosyl hydrolase family 59. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 0.0 | 1 | 499 | 1 | 499 | |
| 0.0 | 1 | 499 | 1 | 499 | |
| 0.0 | 9 | 499 | 1 | 488 | |
| 3.19e-293 | 16 | 497 | 7 | 487 | |
| 3.19e-293 | 16 | 497 | 7 | 487 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.30e-49 | 80 | 493 | 77 | 488 | The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGK_B The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGK_C The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_A The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_B The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron],5NGL_C The endo-beta1,6-glucanase BT3312 [Bacteroides thetaiotaomicron] |
|
| 1.64e-27 | 80 | 473 | 76 | 472 | human acid-beta-glucosidase [Homo sapiens],1OGS_B human acid-beta-glucosidase [Homo sapiens],1Y7V_A Chain A, Glucosylceramidase [Homo sapiens],1Y7V_B Chain B, Glucosylceramidase [Homo sapiens],2F61_A Crystal structure of partially deglycosylated acid beta-glucosidase [Homo sapiens],2F61_B Crystal structure of partially deglycosylated acid beta-glucosidase [Homo sapiens],2J25_A Partially deglycosylated glucoceramidase [Homo sapiens],2J25_B Partially deglycosylated glucoceramidase [Homo sapiens],2NSX_A Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_B Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_C Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NSX_D Structure of acid-beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease [Homo sapiens],2NT0_A Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_B Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_C Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT0_D Acid-beta-glucosidase low pH, glycerol bound [Homo sapiens],2NT1_A Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_B Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_C Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],2NT1_D Structure of acid-beta-glucosidase at neutral pH [Homo sapiens],3GXD_A Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_B Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_C Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXD_D Crystal structure of Apo acid-beta-glucosidase pH 4.5 [Homo sapiens],3GXF_A Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_B Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_C Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXF_D Crystal structure of acid-beta-glucosidase with isofagomine at neutral pH [Homo sapiens],3GXI_A Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_B Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_C Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXI_D Crystal structure of acid-beta-glucosidase at pH 5.5 [Homo sapiens],3GXM_A Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_B Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_C Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3GXM_D Crystal structure of acid-beta-glucosidase at pH 4.5, phosphate crystallization condition [Homo sapiens],3RIK_A The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_B The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_C The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIK_D The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_A The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_B The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_C The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],3RIL_D The acid beta-glucosidase active site exhibits plasticity in binding 3,4,5,6-tetrahydroxyazepane-based inhibitors: implications for pharmacological chaperone design for gaucher disease [Homo sapiens],6MOZ_A Structure of acid-beta-glucosidase in complex with an aromatic pyrrolidine iminosugar inhibitor [Homo sapiens],6MOZ_B Structure of acid-beta-glucosidase in complex with an aromatic pyrrolidine iminosugar inhibitor [Homo sapiens],6Q1N_A Glucocerebrosidase in complex with pharmacological chaperone IMX8 [Homo sapiens],6Q1N_B Glucocerebrosidase in complex with pharmacological chaperone IMX8 [Homo sapiens],6Q1P_A Glucocerebrosidase in complex with pharmacological chaperone norIMX8 [Homo sapiens],6Q1P_B Glucocerebrosidase in complex with pharmacological chaperone norIMX8 [Homo sapiens],6Q6K_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with cyclophellitol activity based probe with Cy5 tag (ME569) [Homo sapiens],6Q6K_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with cyclophellitol activity based probe with Cy5 tag (ME569) [Homo sapiens],6Q6L_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with adamantyl-cyclophellitol inhibitor (ME656) [Homo sapiens],6Q6L_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with adamantyl-cyclophellitol inhibitor (ME656) [Homo sapiens],6Q6N_A Crystal structure of recombinant human beta-glucocerebrosidase in complex with biphenyl-cyclophellitol inhibitor (ME655) [Homo sapiens],6Q6N_B Crystal structure of recombinant human beta-glucocerebrosidase in complex with biphenyl-cyclophellitol inhibitor (ME655) [Homo sapiens],6TJJ_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6TJJ_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YTP_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YTP_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YUT_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YUT_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6YV3_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6YV3_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6Z39_AAA Chain AAA, Glucosylceramidase [Homo sapiens],6Z39_BBB Chain BBB, Glucosylceramidase [Homo sapiens] |
|
| 1.64e-27 | 80 | 473 | 76 | 472 | Velaglucerase alfa [Homo sapiens],2WKL_B Velaglucerase alfa [Homo sapiens],5LVX_A Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_B Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_C Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],5LVX_D Crystal structure of glucocerebrosidase with an inhibitory quinazoline modulator [Homo sapiens],6TJK_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6TJK_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],6TJQ_BBB Chain BBB, Glucosylceramidase [Homo sapiens],6TN1_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6YTR_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],6YTR_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],6Z3I_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens],7NWV_AAA Chain AAA, Lysosomal acid glucosylceramidase [Homo sapiens],7NWV_BBB Chain BBB, Lysosomal acid glucosylceramidase [Homo sapiens] |
|
| 1.75e-27 | 80 | 473 | 78 | 474 | acid-beta-glucosidase with N-butyl-deoxynojirimycin [Homo sapiens],2V3D_B acid-beta-glucosidase with N-butyl-deoxynojirimycin [Homo sapiens],2V3E_A acid-beta-glucosidase with N-nonyl-deoxynojirimycin [Homo sapiens],2V3E_B acid-beta-glucosidase with N-nonyl-deoxynojirimycin [Homo sapiens],2V3F_A acid-beta-glucosidase produced in carrot [Homo sapiens],2V3F_B acid-beta-glucosidase produced in carrot [Homo sapiens],2VT0_A X-ray structure of a conjugate with conduritol-beta-epoxide of acid-beta-glucosidase overexpressed in cultured plant cells [Homo sapiens],2VT0_B X-ray structure of a conjugate with conduritol-beta-epoxide of acid-beta-glucosidase overexpressed in cultured plant cells [Homo sapiens],2WCG_A X-ray structure of acid-beta-glucosidase with N-octyl(cyclic guanidine)-nojirimycin in the active site [Homo sapiens],2WCG_B X-ray structure of acid-beta-glucosidase with N-octyl(cyclic guanidine)-nojirimycin in the active site [Homo sapiens],2XWD_A X-Ray Structure Of Acid-Beta-Glucosidase With 5n,6o-(N'-(N- Octyl)imino)nojirimycin In The Active Site [Homo sapiens],2XWD_B X-Ray Structure Of Acid-Beta-Glucosidase With 5n,6o-(N'-(N- Octyl)imino)nojirimycin In The Active Site [Homo sapiens],2XWE_A X-ray Structure Of Acid-beta-glucosidase With 5n,6s-(n'-(n- Octyl)imino)-6-thionojirimycin In The Active Site [Homo sapiens],2XWE_B X-ray Structure Of Acid-beta-glucosidase With 5n,6s-(n'-(n- Octyl)imino)-6-thionojirimycin In The Active Site [Homo sapiens] |
|
| 2.20e-27 | 80 | 473 | 115 | 511 | Chain A, Glucosylceramidase [Homo sapiens],6T13_B Chain B, Glucosylceramidase [Homo sapiens],6T13_C Chain C, Glucosylceramidase [Homo sapiens],6T13_D Chain D, Glucosylceramidase [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.67e-294 | 16 | 497 | 7 | 487 | Endo-1,6-beta-D-glucanase neg1 OS=Neosartorya fumigata (strain ATCC MYA-4609 / Af293 / CBS 101355 / FGSC A1100) OX=330879 GN=neg1 PE=1 SV=1 |
|
| 1.44e-250 | 12 | 498 | 9 | 490 | Endo-1,6-beta-D-glucanase BGN16.3 OS=Trichoderma harzianum OX=5544 PE=1 SV=1 |
|
| 3.72e-198 | 37 | 497 | 23 | 477 | Endo-1,6-beta-D-glucanase OS=Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) OX=367110 GN=neg-1 PE=1 SV=2 |
|
| 5.92e-33 | 80 | 463 | 97 | 483 | Putative glucosylceramidase 4 OS=Caenorhabditis elegans OX=6239 GN=gba-4 PE=3 SV=2 |
|
| 1.70e-29 | 80 | 463 | 96 | 479 | Putative glucosylceramidase 2 OS=Caenorhabditis elegans OX=6239 GN=gba-2 PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
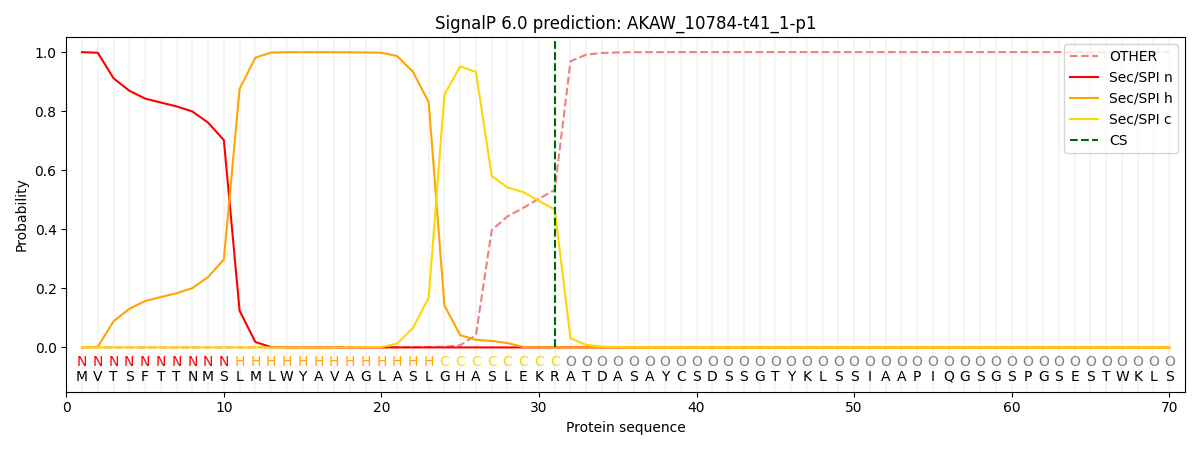
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000509 | 0.999450 | CS pos: 31-32. Pr: 0.4670 |
