You are browsing environment: FUNGIDB
CAZyme Information: AGR57_8985T0-p1
You are here: Home > Sequence: AGR57_8985T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phanerochaete chrysosporium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Agaricomycetes; ; Phanerochaetaceae; Phanerochaete; Phanerochaete chrysosporium | |||||||||||
| CAZyme ID | AGR57_8985T0-p1 | |||||||||||
| CAZy Family | GT2|GT2 | |||||||||||
| CAZyme Description | Multicopper oxidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA1 | 98 | 568 | 3.5e-107 | 0.9804469273743017 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 274555 | ascorbase | 1.87e-83 | 87 | 579 | 1 | 535 | L-ascorbate oxidase, plant type. Members of this protein family are the copper-containing enzyme L-ascorbate oxidase (EC 1.10.3.3), also called ascorbase. This family is found in flowering plants, and shows greater sequence similarity to a family of laccases (EC 1.10.3.2) from plants than to other known ascorbate oxidases. |
| 274556 | laccase | 2.50e-71 | 87 | 570 | 3 | 515 | laccase, plant. Members of this protein family include the copper-containing enzyme laccase (EC 1.10.3.2), often several from a single plant species, and additional, uncharacterized, closely related plant proteins termed laccase-like multicopper oxidases. This protein family shows considerable sequence similarity to the L-ascorbate oxidase (EC 1.10.3.3) family. Laccases are enzymes of rather broad specificity, and classification of all proteins scoring about the trusted cutoff of this model as laccases may be appropriate. |
| 259926 | CuRO_1_Diphenol_Ox | 3.26e-70 | 88 | 209 | 1 | 119 | The first cupredoxin domain of fungal laccase, diphenol oxidase. Diphenol oxidase belongs to the laccase family. It catalyzes the initial steps in melanin biosynthesis from diphenols. Melanin is one of the virulence factors of infectious fungi. In the pathogenesis of C. neoformans, melanin pigments have been shown to protect the fungal cells from oxidative and microbicidal activities of host defense systems. Laccase is a blue multicopper oxidase (MCO) which catalyzes the oxidation of a variety aromatic - notably phenolic and inorganic substances coupled to the reduction of molecular oxygen to water. It has been implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism. Although MCOs have diverse functions, majority of them have three cupredoxin domain repeats that include one mononuclear and one trinuclear copper center. The copper ions are bound in several sites: Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster. MCOs oxidize their substrate by accepting electrons at a mononuclear copper center and transferring them to the active site trinuclear copper center. The cupredoxin domain 1 of 3-domain MCOs contains part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
| 177843 | PLN02191 | 1.18e-66 | 100 | 579 | 36 | 558 | L-ascorbate oxidase |
| 259977 | CuRO_3_MCO_like_4 | 1.34e-66 | 419 | 574 | 1 | 166 | The third cupredoxin domain of uncharacterized multicopper oxidase. Multicopper Oxidases (MCOs) are multi-domain enzymes that are able to couple oxidation of substrates with reduction of dioxygen to water. MCOs oxidize their substrate by accepting electrons at a mononuclear copper centre and transferring them to a trinuclear copper centre which binds a dioxygen. The dioxygen, following the transfer of four electrons, is reduced to two molecules of water. These MCOs are capable of oxidizing a vast range of substrates, varying from aromatic to inorganic compounds such as metals. This subfamily of MCOs is composed of three cupredoxin domains. The cupredoxin domain 3 of 3-domain MCOs contains the Type 1 (T1) copper binding site and part the trinuclear copper binding site, which is located at the interface of domains 1 and 3. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 3.75e-155 | 65 | 593 | 92 | 619 | |
| 5.71e-151 | 75 | 592 | 86 | 604 | |
| 1.03e-149 | 48 | 592 | 61 | 614 | |
| 1.17e-127 | 84 | 594 | 108 | 660 | |
| 5.76e-124 | 86 | 592 | 121 | 655 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4.65e-74 | 94 | 568 | 11 | 462 | Chain A, LACCASE 1 [Coprinopsis cinerea] |
|
| 4.76e-74 | 94 | 568 | 11 | 462 | Chain A, Laccase [Coprinopsis cinerea] |
|
| 7.84e-70 | 75 | 594 | 15 | 509 | The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The fifth structure of the series with total exposition time 123 min. [Steccherinum murashkinskyi],5MHX_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The sixth structure of the series with total exposition time 153 min. [Steccherinum murashkinskyi],5MHY_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The seventh structure of the series with total exposition time 183 min. [Steccherinum murashkinskyi],5MHZ_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 8-th structure of the series with total exposition time 213 min. [Steccherinum murashkinskyi],5MI1_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 9-th structure of the series with total exposition time 243 min. [Steccherinum murashkinskyi],5MI2_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 10-th structure of the series with total exposition time 273 min. [Steccherinum murashkinskyi],5MIA_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 11-th structure of the series with total exposition time 303 min. [Steccherinum murashkinskyi],5MIB_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 12-th structure of the series with total exposition time 333 min. [Steccherinum murashkinskyi],5MIC_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 13-th structure of the series with total exposition time 363 min. [Steccherinum murashkinskyi],5MID_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 14-th structure of the series with total exposition time 393 min. [Steccherinum murashkinskyi],5MIE_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 15-th structure of the series with total exposition time 423 min. [Steccherinum murashkinskyi],5MIG_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The 16-th structure of the series with total exposition time 453 min. The crystal was quick refreezing before this data collection. [Steccherinum murashkinskyi] |
|
| 1.34e-69 | 94 | 594 | 11 | 490 | Single crystal serial study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi at sub-atomic resolution. Third structure of the series with 315 KGy dose. [Steccherinum murashkinskyi],6RHI_A Single crystal serial study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi at sub-atomic resolution. Ninth structure of the series with 1215 KGy dose. [Steccherinum murashkinskyi],6RHO_A Single crystal serial study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi at sub-atomic resolution. Twentieth structure of the series with 4065 KGy dose. [Steccherinum murashkinskyi],6RHP_A Single crystal serial study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi at sub-atomic resolution. Twenty first structure of the series with 4415 KGy dose (collected after refreezing). [Steccherinum murashkinskyi],6RHR_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by chloride anions at sub-atomic resolution. First structure of the series with 15 KGy dose. [Steccherinum murashkinskyi],6RHU_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by chloride anions at sub-atomic resolution. Second structure of the series with 165 KGy dose. [Steccherinum murashkinskyi],6RHX_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by chloride anions at sub-atomic resolution. Third structure of the series with 315 KGy dose. [Steccherinum murashkinskyi],6RI0_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by chloride anions at sub-atomic resolution. Ninth structure of the series with 1215 KGy dose. [Steccherinum murashkinskyi],6RI2_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by chloride anions at sub-atomic resolution. Twentieth structure of the series with 4065 KGy dose. [Steccherinum murashkinskyi],6RI4_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. First structure of the series with 13 KGy dose. [Steccherinum murashkinskyi],6RI6_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. Second structure of the series with 400 KGy dose. [Steccherinum murashkinskyi],6RI8_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. Third structure of the series with 800 KGy dose. [Steccherinum murashkinskyi],6RII_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. Fourth structure of the series with 1200 KGy dose. [Steccherinum murashkinskyi],6RIK_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. Thirteenth structure of the series with 5200 KGy dose. [Steccherinum murashkinskyi],6RIL_A Single crystal serial study of the inhibition of laccases from Steccherinum murashkinskyi by fluoride anions at sub-atomic resolution. Fourteenth structure of the series with 5600 KGy dose (data was collected after refreezing). [Steccherinum murashkinskyi] |
|
| 1.37e-69 | 94 | 594 | 11 | 490 | Structural study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi. First structure of the series with 3 min total X-ray exposition time. [Steccherinum murashkinskyi],5MHU_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The third structure of the series with total exposition time 63 min. [Steccherinum murashkinskyi],5MHV_A The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi.The fourth structure of the series with total exposition time 93 min. [Steccherinum murashkinskyi] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.85e-75 | 85 | 593 | 59 | 579 | Laccase-1 OS=Cryptococcus neoformans var. neoformans serotype D (strain B-3501A) OX=283643 GN=LAC1 PE=1 SV=1 |
|
| 3.31e-75 | 87 | 576 | 21 | 489 | Laccase-2 OS=Agaricus bisporus OX=5341 GN=lcc2 PE=1 SV=1 |
|
| 3.60e-75 | 85 | 593 | 59 | 579 | Laccase-1 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC1 PE=1 SV=1 |
|
| 1.92e-74 | 85 | 593 | 59 | 579 | Laccase-2 OS=Cryptococcus neoformans var. grubii serotype A (strain H99 / ATCC 208821 / CBS 10515 / FGSC 9487) OX=235443 GN=LAC2 PE=3 SV=2 |
|
| 2.64e-72 | 93 | 571 | 68 | 518 | Laccase-1 OS=Botryotinia fuckeliana OX=40559 GN=lcc1 PE=2 SV=3 |
SignalP and Lipop Annotations help
This protein is predicted as SP
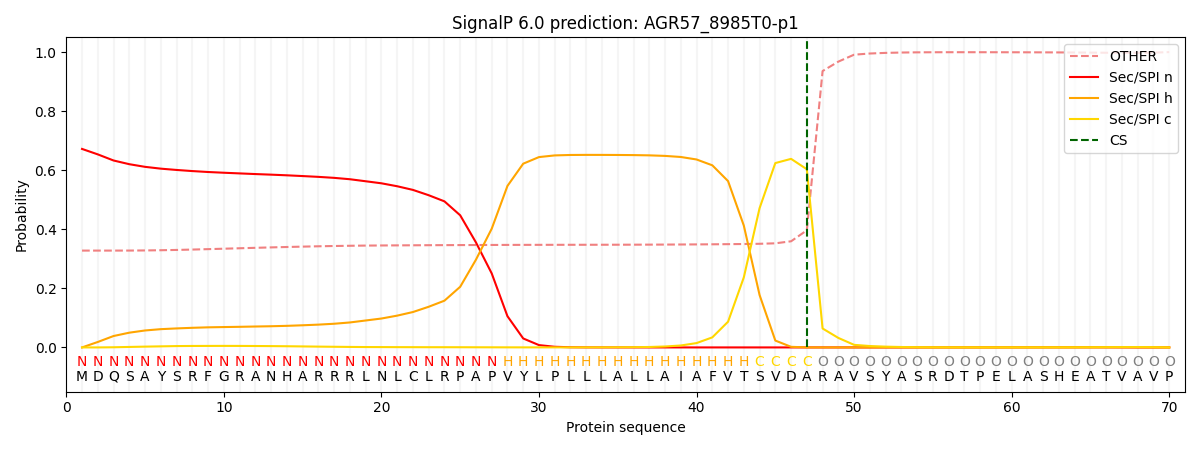
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.340252 | 0.659729 | CS pos: 47-48. Pr: 0.6033 |

