You are browsing environment: FUNGIDB
CAZyme Information: AGR57_10276T0-p1
You are here: Home > Sequence: AGR57_10276T0-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
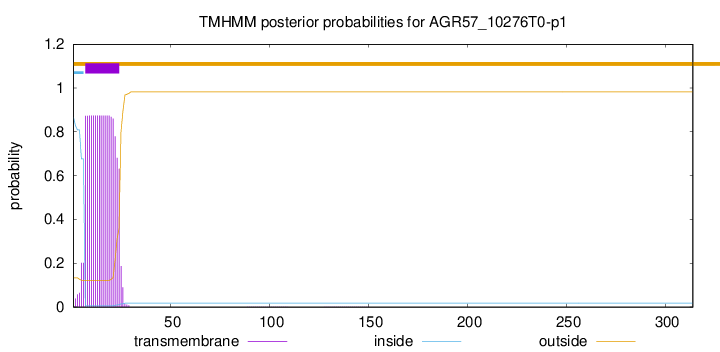
TMHMM annotations
Basic Information help
| Species | Phanerochaete chrysosporium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Basidiomycota; Agaricomycetes; ; Phanerochaetaceae; Phanerochaete; Phanerochaete chrysosporium | |||||||||||
| CAZyme ID | AGR57_10276T0-p1 | |||||||||||
| CAZy Family | AA14 | |||||||||||
| CAZyme Description | Class II peroxidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
Enzyme Prediction help
| EC | 1.11.1.16:9 | 1.11.1.13:5 | 1.11.1.-:1 |
|---|
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 44 | 298 | 2.7e-89 | 0.996078431372549 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 173826 | ligninase | 1.78e-132 | 28 | 311 | 1 | 289 | Ligninase and other manganese-dependent fungal peroxidases. Ligninases and related extracellular fungal peroxidases belong to class II of the plant heme-dependent peroxidase superfamily. All members of the superfamily share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Class II peroxidases are fungal glycoproteins that have been implicated in the oxidative breakdown of lignin, the main cell wall component of woody plants. They contain four conserved disulphide bridges and two conserved calcium binding sites. |
| 173823 | plant_peroxidase_like | 6.12e-40 | 49 | 293 | 1 | 254 | Heme-dependent peroxidases similar to plant peroxidases. Along with animal peroxidases, these enzymes belong to a group of peroxidases containing a heme prosthetic group (ferriprotoporphyrin IX), which catalyzes a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. The plant peroxidase-like superfamily is found in all three kingdoms of life and carries out a variety of biosynthetic and degradative functions. Several sub-families can be identified. Class I includes intracellular peroxidases present in fungi, plants, archaea and bacteria, called catalase-peroxidases, that can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. Catalase-peroxidases are typically comprised of two homologous domains that probably arose via a single gene duplication event. Class II includes ligninase and other extracellular fungal peroxidases, while class III is comprised of classic extracellular plant peroxidases, like horseradish peroxidase. |
| 395089 | peroxidase | 2.18e-27 | 47 | 277 | 1 | 187 | Peroxidase. |
| 173825 | ascorbate_peroxidase | 9.05e-26 | 47 | 296 | 16 | 249 | Ascorbate peroxidases and cytochrome C peroxidases. Ascorbate peroxidases are a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Along with related catalase-peroxidases, ascorbate peroxidases belong to class I of the plant superfamily. Ascorbate peroxidases are found in the chloroplasts and/or cytosol of algae and plants, where they have been shown to control the concentration of lethal hydrogen peroxide molecules. The yeast cytochrome c peroxidase is a divergent member of the family; it forms a complex with cytochrome c to catalyze the reduction of hydrogen peroxide to water. |
| 173827 | secretory_peroxidase | 8.01e-19 | 124 | 296 | 94 | 282 | Horseradish peroxidase and related secretory plant peroxidases. Secretory peroxidases belong to class III of the plant heme-dependent peroxidase superfamily. All members of the superfamily share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Class III peroxidases are found in the extracellular space or in the vacuole in plants where they have been implicated in hydrogen peroxide detoxification, auxin catabolism and lignin biosynthesis, and stress response. Class III peroxidases contain four conserved disulphide bridges and two conserved calcium binding sites. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 4.83e-225 | 1 | 314 | 1 | 315 | |
| 1.22e-218 | 1 | 313 | 1 | 314 | |
| 6.30e-218 | 1 | 311 | 1 | 312 | |
| 4.18e-104 | 6 | 311 | 28 | 342 | |
| 8.17e-98 | 18 | 310 | 26 | 323 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2.11e-95 | 29 | 310 | 3 | 290 | CRYSTAL STRUCTURE OF MANGANESE PEROXIDASE 4 FROM PLEUROTUS OSTREATUS - CRYSTAL FORM I [Pleurotus ostreatus],4BM1_B CRYSTAL STRUCTURE OF MANGANESE PEROXIDASE 4 FROM PLEUROTUS OSTREATUS - CRYSTAL FORM I [Pleurotus ostreatus],4BM2_A Crystal Structure Of Manganese Peroxidase 4 From Pleurotus Ostreatus - Crystal Form Ii [Pleurotus ostreatus],4BM3_A Crystal Structure Of Manganese Peroxidase 4 From Pleurotus Ostreatus - Crystal Form Iii [Pleurotus ostreatus],4BM4_A Crystal Structure Of Manganese Peroxidase 4 From Pleurotus Ostreatus - Crystal Form Iv [Pleurotus ostreatus] |
|
| 2.10e-90 | 30 | 310 | 3 | 283 | Site-Directed Mutagenesis of the Catalytic Tryptophan Environment in Pleurotus eryngii Versatile Peroxidase [Pleurotus eryngii] |
|
| 5.59e-90 | 30 | 310 | 3 | 283 | The crystal structures of several mutants of pleurotus eryngii versatile peroxidase [Pleurotus eryngii] |
|
| 5.95e-90 | 30 | 310 | 3 | 283 | The crystal structures of several mutants of Pleurotus eryngii versatile peroxidase [Pleurotus eryngii] |
|
| 6.55e-90 | 30 | 310 | 3 | 283 | Chain A, Versatile peroxidase VPL2 [Pleurotus eryngii] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 9.40e-91 | 17 | 310 | 20 | 313 | Versatile peroxidase VPL1 OS=Pleurotus eryngii OX=5323 GN=vpl1 PE=1 SV=1 |
|
| 2.66e-90 | 17 | 310 | 20 | 313 | Versatile peroxidase VPL2 OS=Pleurotus eryngii OX=5323 GN=vpl2 PE=1 SV=1 |
|
| 3.66e-88 | 16 | 310 | 14 | 317 | Peroxidase OS=Coprinopsis cinerea OX=5346 GN=CIP1 PE=1 SV=2 |
|
| 7.56e-88 | 16 | 310 | 14 | 318 | Peroxidase OS=Arthromyces ramosus OX=5451 PE=1 SV=3 |
|
| 1.47e-87 | 16 | 310 | 14 | 317 | Peroxidase OS=Coprinopsis cinerea (strain Okayama-7 / 130 / ATCC MYA-4618 / FGSC 9003) OX=240176 GN=CIP1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
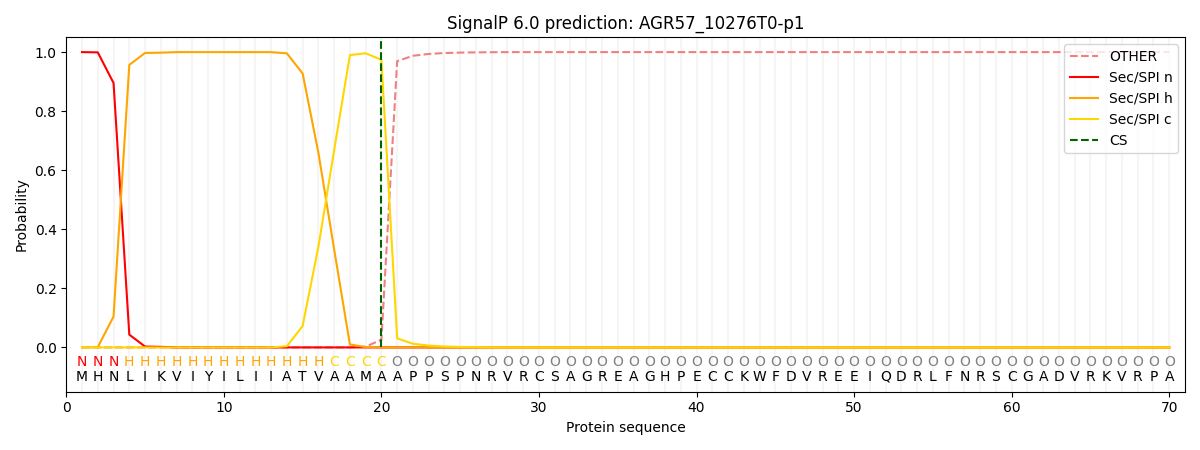
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.000184 | 0.999800 | CS pos: 20-21. Pr: 0.9739 |