You are browsing environment: FUNGIDB
CAZyme Information: ACLA_063890-t26_1-p1
You are here: Home > Sequence: ACLA_063890-t26_1-p1
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Aspergillus clavatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Ascomycota; Eurotiomycetes; ; Aspergillaceae; Aspergillus; Aspergillus clavatus | |||||||||||
| CAZyme ID | ACLA_063890-t26_1-p1 | |||||||||||
| CAZy Family | GH5 | |||||||||||
| CAZyme Description | FAD binding domain protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | ||||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA7 | 58 | 490 | 1.8e-60 | 0.9737991266375546 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| 223354 | GlcD | 2.21e-20 | 69 | 489 | 35 | 450 | FAD/FMN-containing dehydrogenase [Energy production and conversion]. |
| 396238 | FAD_binding_4 | 1.36e-14 | 68 | 202 | 3 | 139 | FAD binding domain. This family consists of various enzymes that use FAD as a co-factor, most of the enzymes are similar to oxygen oxidoreductase. One of the enzymes Vanillyl-alcohol oxidase (VAO) has a solved structure, the alignment includes the FAD binding site, called the PP-loop, between residues 99-110. The FAD molecule is covalently bound in the known structure, however the residue that links to the FAD is not in the alignment. VAO catalyzes the oxidation of a wide variety of substrates, ranging form aromatic amines to 4-alkylphenols. Other members of this family include D-lactate dehydrogenase, this enzyme catalyzes the conversion of D-lactate to pyruvate using FAD as a co-factor; mitomycin radical oxidase, this enzyme oxidizes the reduced form of mitomycins and is involved in mitomycin resistance. This family includes MurB an UDP-N-acetylenolpyruvoylglucosamine reductase enzyme EC:1.1.1.158. This enzyme is involved in the biosynthesis of peptidoglycan. |
| 223882 | MurB | 0.001 | 69 | 152 | 24 | 106 | UDP-N-acetylenolpyruvoylglucosamine reductase [Cell wall/membrane/envelope biogenesis]. |
| 215242 | PLN02441 | 0.002 | 68 | 225 | 67 | 233 | cytokinin dehydrogenase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| 4.18e-15 | 72 | 231 | 69 | 230 | |
| 1.59e-14 | 69 | 231 | 62 | 227 | |
| 1.65e-14 | 69 | 231 | 51 | 214 | |
| 1.65e-14 | 69 | 231 | 51 | 214 | |
| 5.17e-14 | 69 | 231 | 51 | 214 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1.21e-20 | 57 | 236 | 36 | 214 | The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_B The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_C The crystal structure of EncM T139V mutant [Streptomyces maritimus],6FYD_D The crystal structure of EncM T139V mutant [Streptomyces maritimus] |
|
| 2.17e-20 | 57 | 236 | 36 | 214 | The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_B The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_C The crystal structure of EncM V135M mutant [Streptomyces maritimus],6FYF_D The crystal structure of EncM V135M mutant [Streptomyces maritimus] |
|
| 2.17e-20 | 57 | 236 | 36 | 214 | The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_B The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_C The crystal structure of EncM V135T mutant [Streptomyces maritimus],6FYG_D The crystal structure of EncM V135T mutant [Streptomyces maritimus] |
|
| 2.91e-20 | 57 | 236 | 36 | 214 | The crystal structure of EncM H138T mutant [Streptomyces maritimus],6FYE_B The crystal structure of EncM H138T mutant [Streptomyces maritimus] |
|
| 3.90e-20 | 57 | 236 | 36 | 214 | Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_B Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_C Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],4XLO_D Crystal Structure of EncM (crystallized with 4 mM NADPH) [Streptomyces maritimus],6FOQ_A The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_B The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_C The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOQ_D The crystal structure of EncM complexed with dioxygen under 15 bar of oxygen pressure. [Streptomyces maritimus],6FOW_A The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_B The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_C The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FOW_D The crystal structure of EncM complexed with dioxygen under 10 bar of oxygen pressure. [Streptomyces maritimus],6FP3_A The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_B The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_C The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FP3_D The crystal structure of EncM complexed with dioxygen under 5 bar of oxygen pressure. [Streptomyces maritimus],6FY8_A The crystal structure of EncM bromide soak [Streptomyces maritimus],6FY9_A The crystal structure of EncM complex with xenon under 15 bars Xe pressure [Streptomyces maritimus],6FYA_A The crystal structure of EncM under anaerobic conditions [Streptomyces maritimus],6FYA_B The crystal structure of EncM under anaerobic conditions [Streptomyces maritimus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5.39e-126 | 20 | 499 | 21 | 507 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti OX=5082 GN=prx3 PE=3 SV=1 |
|
| 5.39e-126 | 20 | 499 | 21 | 507 | FAD-dependent monooxygenase prx3 OS=Penicillium roqueforti (strain FM164) OX=1365484 GN=prx3 PE=3 SV=1 |
|
| 5.47e-123 | 30 | 498 | 31 | 506 | FAD-dependent monooxygenase prx3 OS=Penicillium rubens (strain ATCC 28089 / DSM 1075 / NRRL 1951 / Wisconsin 54-1255) OX=500485 GN=prx3 PE=2 SV=1 |
|
| 2.67e-64 | 59 | 499 | 88 | 514 | Bifunctional solanapyrone synthase OS=Alternaria solani OX=48100 GN=sol5 PE=1 SV=1 |
|
| 1.22e-63 | 46 | 499 | 61 | 513 | FAD-dependent monooxygenase drtC OS=Aspergillus calidoustus OX=454130 GN=drtC PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
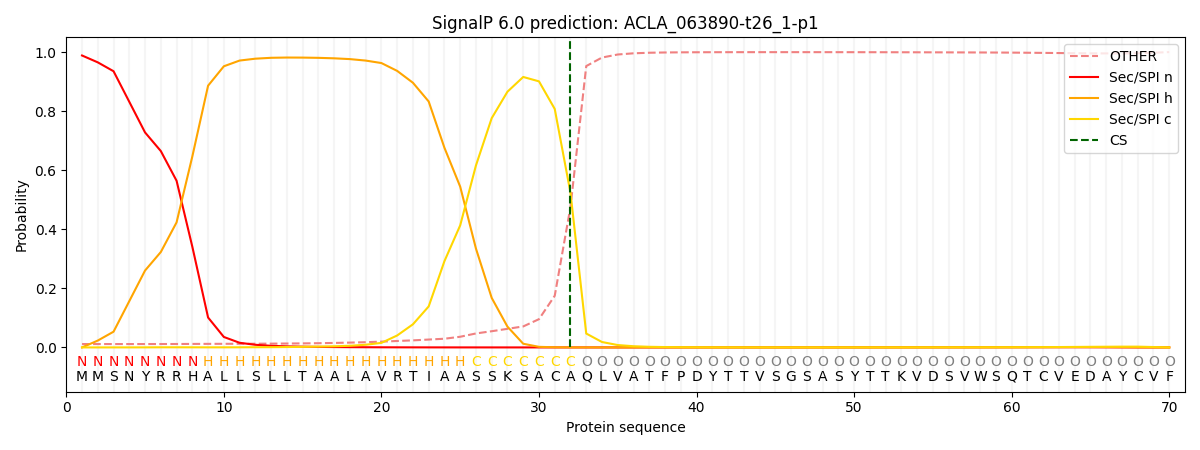
| Other | SP_Sec_SPI | CS Position |
|---|---|---|
| 0.015784 | 0.984179 | CS pos: 32-33. Pr: 0.5234 |
