You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004516_00769
You are here: Home > Sequence: MGYG000004516_00769
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
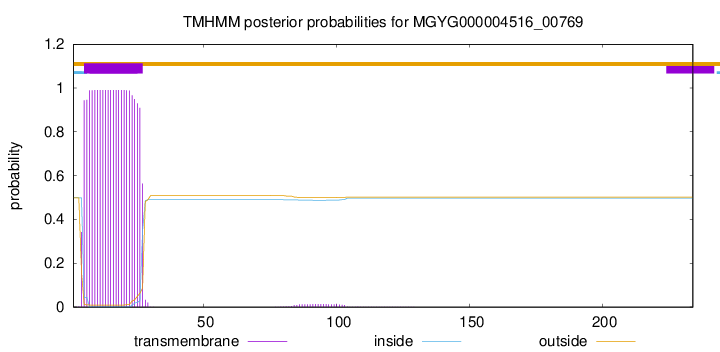
TMHMM annotations
Basic Information help
| Species | Sneathia amnii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Fusobacteriota; Fusobacteriia; Fusobacteriales; Leptotrichiaceae; Sneathia; Sneathia amnii | |||||||||||
| CAZyme ID | MGYG000004516_00769 | |||||||||||
| CAZy Family | GH25 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 24453; End: 25157 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH25 | 41 | 210 | 1.2e-44 | 0.9943502824858758 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06413 | GH25_muramidase_1 | 3.44e-86 | 36 | 219 | 1 | 189 | Uncharacterized bacterial muramidase containing a glycosyl hydrolase family 25 (GH25) catalytic domain. Endo-N-acetylmuramidases are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. |
| cd06524 | GH25_YegX-like | 8.31e-51 | 39 | 220 | 1 | 193 | YegX is an uncharacterized bacterial protein with a glycosyl hydrolase family 25 (GH25) catalytic domain that is similar in sequence to the CH-type (Chalaropsis-type) lysozymes of the GH25 family of endolysins. |
| cd00599 | GH25_muramidase | 3.73e-45 | 39 | 218 | 1 | 185 | Endo-N-acetylmuramidases (muramidases) are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. This family of muramidases contains a glycosyl hydrolase family 25 (GH25) catalytic domain and is found in bacteria, fungi, slime molds, round worms, protozoans and bacteriophages. The bacteriophage members are referred to as endolysins which are involved in lysing the host cell at the end of the replication cycle to allow release of mature phage particles. Endolysins are typically modular enzymes consisting of a catalytically active domain that hydrolyzes the peptidoglycan cell wall and a cell wall-binding domain that anchors the protein to the cell wall. Endolysins generally have narrow substrate specificities with either intra-species or intra-genus bacteriolytic activity. |
| COG3757 | Acm | 3.43e-41 | 22 | 220 | 44 | 254 | Lyzozyme M1 (1,4-beta-N-acetylmuramidase), GH25 family [Cell wall/membrane/envelope biogenesis]. |
| pfam01183 | Glyco_hydro_25 | 2.66e-38 | 41 | 210 | 1 | 180 | Glycosyl hydrolases family 25. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AKC95719.1 | 5.27e-172 | 1 | 234 | 1 | 234 |
| BBM36160.1 | 1.64e-97 | 3 | 234 | 6 | 235 |
| ALA95773.1 | 1.82e-96 | 1 | 220 | 1 | 223 |
| ACZ00521.1 | 1.95e-93 | 20 | 230 | 21 | 228 |
| AVL43061.1 | 1.95e-93 | 20 | 230 | 21 | 228 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2WAG_A | 9.03e-44 | 36 | 223 | 14 | 210 | TheStructure of a family 25 Glycosyl hydrolase from Bacillus anthracis. [Bacillus anthracis str. Ames] |
| 4KRU_A | 5.14e-17 | 40 | 217 | 22 | 205 | X-raystructure of catalytic domain of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101] |
| 4KRT_A | 2.15e-16 | 40 | 217 | 22 | 205 | X-raystructure of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101],4KRT_B X-ray structure of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101] |
| 1JFX_A | 7.99e-16 | 38 | 217 | 5 | 200 | Crystalstructure of the bacterial lysozyme from Streptomyces coelicolor at 1.65 A resolution [Streptomyces coelicolor] |
| 5JIP_A | 3.90e-12 | 40 | 217 | 16 | 220 | Crystalstructure of the Clostridium perfringens spore cortex lytic enzyme SleM [Clostridium perfringens],5JIP_B Crystal structure of the Clostridium perfringens spore cortex lytic enzyme SleM [Clostridium perfringens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8FFY2 | 6.90e-32 | 34 | 218 | 63 | 253 | Uncharacterized protein YegX OS=Escherichia coli O6:H1 (strain CFT073 / ATCC 700928 / UPEC) OX=199310 GN=yegX PE=3 SV=2 |
| P76421 | 1.36e-31 | 34 | 218 | 63 | 253 | Uncharacterized protein YegX OS=Escherichia coli (strain K12) OX=83333 GN=yegX PE=3 SV=2 |
| Q8X7H0 | 3.76e-31 | 34 | 218 | 63 | 253 | Uncharacterized protein YegX OS=Escherichia coli O157:H7 OX=83334 GN=yegX PE=3 SV=2 |
| P26836 | 3.84e-19 | 32 | 217 | 4 | 194 | Probable autolytic lysozyme OS=Clostridium perfringens (strain 13 / Type A) OX=195102 GN=lyc PE=3 SV=2 |
| P25310 | 6.52e-15 | 33 | 217 | 77 | 277 | Lysozyme M1 OS=Streptomyces globisporus OX=1908 GN=acm PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.067584 | 0.927319 | 0.004179 | 0.000285 | 0.000276 | 0.000312 |