You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003948_00511
You are here: Home > Sequence: MGYG000003948_00511
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Rikenellaceae; Alistipes; | |||||||||||
| CAZyme ID | MGYG000003948_00511 | |||||||||||
| CAZy Family | GH76 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 35636; End: 36856 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH76 | 71 | 368 | 9.4e-64 | 0.8128491620111732 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam03663 | Glyco_hydro_76 | 9.85e-23 | 82 | 300 | 34 | 257 | Glycosyl hydrolase family 76. Family of alpha-1,6-mannanases. |
| COG4833 | COG4833 | 2.51e-13 | 139 | 368 | 88 | 326 | Predicted alpha-1,6-mannanase, GH76 family [Carbohydrate transport and metabolism]. |
| COG1331 | YyaL | 4.88e-10 | 145 | 372 | 274 | 520 | Uncharacterized conserved protein YyaL, SSP411 family, contains thoiredoxin and six-hairpin glycosidase-like domains [General function prediction only]. |
| COG1331 | YyaL | 4.06e-04 | 203 | 347 | 413 | 564 | Uncharacterized conserved protein YyaL, SSP411 family, contains thoiredoxin and six-hairpin glycosidase-like domains [General function prediction only]. |
| cd04792 | LanM-like | 0.003 | 42 | 175 | 552 | 682 | Cyclases involved in the biosynthesis of class II lantibiotics, and similar proteins. LanM-like proteins. LanM is a bifunctional enzyme, involved in the synthesis of class II lantibiotics. It is responsible for both the dehydration and the cyclization of the precursor-peptide during lantibiotic synthesis. The C-terminal domain shows similarity to LanC, the cyclase component of the lan operon, but the N terminus seems to be unrelated to the dehydratase, LanB. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AFL76817.1 | 1.83e-130 | 8 | 406 | 3 | 392 |
| QUT74817.1 | 6.17e-129 | 21 | 404 | 15 | 394 |
| BBL09626.1 | 1.04e-126 | 8 | 406 | 3 | 393 |
| BBL12420.1 | 1.04e-126 | 8 | 406 | 3 | 393 |
| BBL01753.1 | 1.47e-126 | 8 | 406 | 3 | 393 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4MU9_A | 3.37e-86 | 35 | 403 | 8 | 364 | Crystalstructure of a putative glycosylhydrolase (BT_3782) from Bacteroides thetaiotaomicron VPI-5482 at 1.89 A resolution [Bacteroides thetaiotaomicron VPI-5482],4MU9_B Crystal structure of a putative glycosylhydrolase (BT_3782) from Bacteroides thetaiotaomicron VPI-5482 at 1.89 A resolution [Bacteroides thetaiotaomicron VPI-5482] |
| 4C1S_A | 2.73e-56 | 83 | 405 | 45 | 371 | Glycosidehydrolase family 76 (mannosidase) Bt3792 from Bacteroides thetaiotaomicron VPI-5482 [Bacteroides thetaiotaomicron VPI-5482],4C1S_B Glycoside hydrolase family 76 (mannosidase) Bt3792 from Bacteroides thetaiotaomicron VPI-5482 [Bacteroides thetaiotaomicron VPI-5482] |
| 6U4Z_A | 2.20e-48 | 83 | 351 | 169 | 451 | CrystalStructure of a family 76 glycoside hydrolase from a bovine Bacteroides thetaiotaomicron strain [Bacteroides thetaiotaomicron] |
| 4V1S_A | 1.62e-29 | 140 | 341 | 139 | 359 | Structureof the GH76 alpha-mannanase BT2949 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],4V1S_B Structure of the GH76 alpha-mannanase BT2949 from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482] |
| 4V1R_A | 7.29e-28 | 140 | 341 | 139 | 359 | Structureof a selenomethionine derivative of the GH76 alpha- mannanase BT2949 Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],4V1R_B Structure of a selenomethionine derivative of the GH76 alpha- mannanase BT2949 Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as LIPO
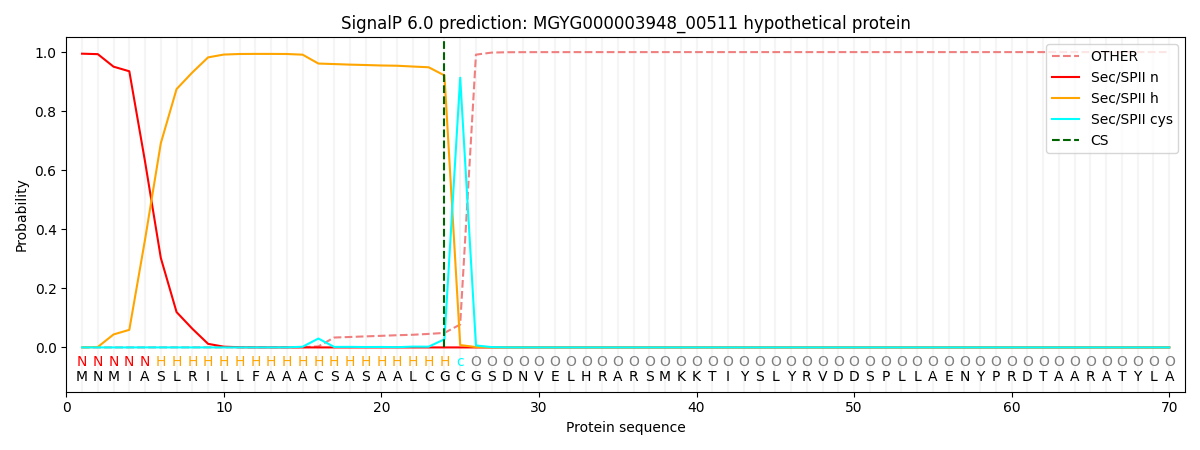
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000042 | 0.005066 | 0.994920 | 0.000002 | 0.000003 | 0.000002 |
