You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003448_00178
You are here: Home > Sequence: MGYG000003448_00178
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | RC9 sp900767405 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; UBA932; RC9; RC9 sp900767405 | |||||||||||
| CAZyme ID | MGYG000003448_00178 | |||||||||||
| CAZy Family | GH20 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 5780; End: 7873 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH20 | 243 | 653 | 4e-91 | 0.9554896142433235 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06569 | GH20_Sm-chitobiase-like | 1.56e-159 | 238 | 675 | 1 | 441 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| cd06563 | GH20_chitobiase-like | 6.98e-94 | 244 | 654 | 3 | 345 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This GH20 domain family includes an N-acetylglucosamidase (GlcNAcase A) from Pseudoalteromonas piscicida and an N-acetylhexosaminidase (SpHex) from Streptomyces plicatus. SpHex lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| pfam00728 | Glyco_hydro_20 | 8.36e-92 | 244 | 653 | 3 | 345 | Glycosyl hydrolase family 20, catalytic domain. This domain has a TIM barrel fold. |
| COG3525 | Chb | 6.47e-77 | 169 | 651 | 186 | 613 | N-acetyl-beta-hexosaminidase [Carbohydrate transport and metabolism]. |
| cd06568 | GH20_SpHex_like | 5.07e-62 | 244 | 651 | 3 | 314 | A subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the N-acetylhexosaminidase from Streptomyces plicatus (SpHex). SpHex catalyzes the hydrolysis of N-acetyl-beta-hexosaminides. An Asp residue within the active site plays a critical role in substrate-assisted catalysis by orienting the 2-acetamido group and stabilizing the transition state. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. Proteins belonging to this subgroup lack the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANU58708.1 | 1.69e-144 | 2 | 692 | 3 | 763 |
| QQR16377.1 | 1.69e-144 | 2 | 692 | 3 | 763 |
| QIU93708.1 | 2.53e-143 | 193 | 692 | 259 | 762 |
| QUT26545.1 | 4.01e-142 | 15 | 692 | 17 | 763 |
| QUR45222.1 | 1.58e-141 | 15 | 692 | 17 | 763 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1QBA_A | 8.61e-73 | 190 | 694 | 258 | 790 | BACTERIALCHITOBIASE, GLYCOSYL HYDROLASE FAMILY 20 [Serratia marcescens],1QBB_A BACTERIAL CHITOBIASE COMPLEXED WITH CHITOBIOSE (DINAG) [Serratia marcescens] |
| 1C7T_A | 2.94e-71 | 190 | 694 | 258 | 790 | ChainA, BETA-N-ACETYLHEXOSAMINIDASE [Serratia marcescens] |
| 1C7S_A | 1.46e-70 | 190 | 694 | 258 | 790 | ChainA, BETA-N-ACETYLHEXOSAMINIDASE [Serratia marcescens] |
| 6EZR_A | 1.16e-68 | 192 | 696 | 209 | 639 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZR_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZS_A Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6EZS_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6K35_A Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi],6K35_B Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi] |
| 6EZT_A | 1.49e-67 | 192 | 696 | 206 | 636 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi],6EZT_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q04786 | 1.08e-84 | 152 | 694 | 225 | 778 | Beta-hexosaminidase OS=Vibrio vulnificus OX=672 GN=hex PE=3 SV=1 |
| P13670 | 6.17e-77 | 192 | 694 | 281 | 815 | N,N'-diacetylchitobiase OS=Vibrio harveyi OX=669 GN=chb PE=1 SV=1 |
| P49007 | 1.88e-75 | 40 | 697 | 59 | 771 | Beta-hexosaminidase B OS=Pseudoalteromonas piscicida OX=43662 GN=nag096 PE=3 SV=1 |
| Q54468 | 6.83e-72 | 190 | 694 | 285 | 817 | Chitobiase OS=Serratia marcescens OX=615 GN=chb PE=1 SV=1 |
| P96155 | 7.24e-66 | 121 | 646 | 116 | 600 | Beta-hexosaminidase OS=Vibrio furnissii OX=29494 GN=exoI PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
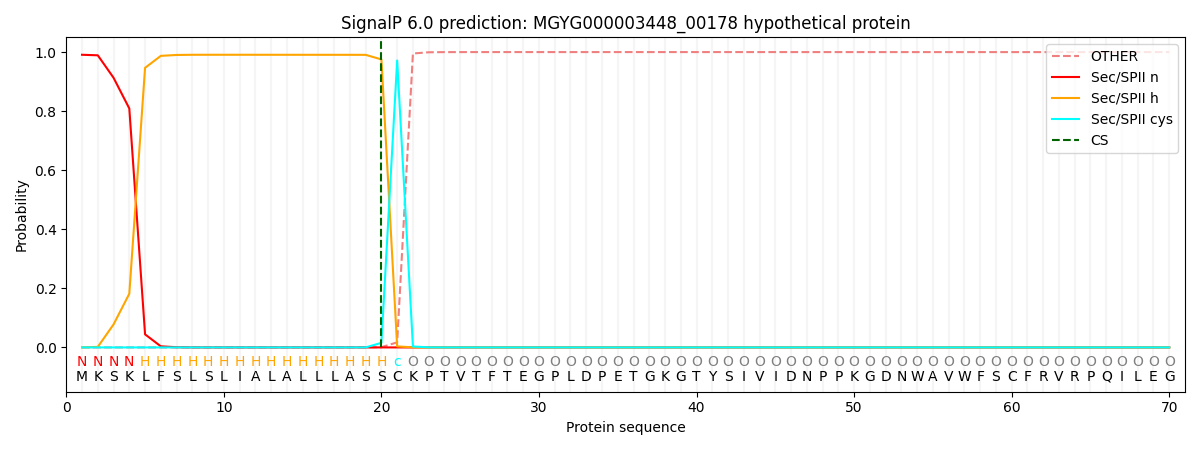
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000012 | 0.008740 | 0.991289 | 0.000003 | 0.000004 | 0.000003 |
