You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000067_01868
You are here: Home > Sequence: MGYG000000067_01868
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
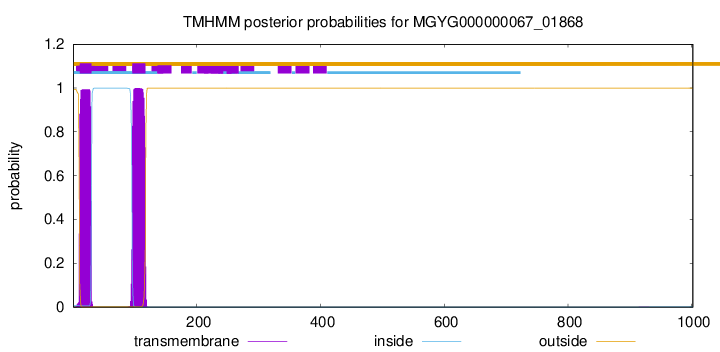
TMHMM annotations
Basic Information help
| Species | Lachnoclostridium_B sp900066765 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Lachnoclostridium_B; Lachnoclostridium_B sp900066765 | |||||||||||
| CAZyme ID | MGYG000000067_01868 | |||||||||||
| CAZy Family | GT4 | |||||||||||
| CAZyme Description | Glycosyltransferase Gtf1 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 98061; End: 101069 Strand: + | |||||||||||
Full Sequence Download help
| MSIDKGKIGK LIGITVAVMS AACVSLLLIA KNKKANYVYE DEPEEKNPLE GKKVVFIEDE | 60 |
| NDAVNADGVK GHLEVVGEEK YNPGFYDKYI KRGLDIFLSF GGLVVLSPIM GTIALAIKME | 120 |
| DPGPVLFTQK RIGQNKKYFK LHKFRSMRLD TPHDVPTHQL ERPDQYITKV GKFIRAHSLD | 180 |
| ELPQIWDIFI GNMSVIGPRP ALWNQDVLTA ERDKYGANDV KPGLTGWAQI NGRDELEIAD | 240 |
| KARLDGEYVE KLSLVMDVKC FLGSLHVFRS DDSVVEGGTG EIKKVGRHYT ESKSDAELIG | 300 |
| KIGFGETVSV DRDAHKKVLI TGAGSYIGES FKAYAEKNYS GNFSVDSVDM IDGSWRERDF | 360 |
| SAYDIVYHVA GIAHADVGNV SDETKEKYYA VNTDLAVEVC QKAKAEGVKE FLFMSSMIVY | 420 |
| GESAPFGVKK IVDEHTVPNP ANFYGDSKLQ ADAGVRELAD DNFKVIVLRP PMIYGKGSKG | 480 |
| NYPTLAKFAK KLPIFPKVKN ERSMLYIENL CEFLCQTMLV DVEENAVVLL PQNGEWTKTS | 540 |
| EMVEEIAEVS GKKIWEIGVL NPAVSVASRI PGKIGGLVNK AFGNMAYAHE MSEYLGMDYQ | 600 |
| VVGLRESIMR TEGTVNEPNN RPKALMLASV ASMIDLFNTD NINILLKLDY DVDVATNFEF | 660 |
| GSITSQVRVN EYKKELIDKG IGVYHIPIPR SLSMVKEIID SYNMVKKLVD EKCYQVVHCH | 720 |
| SPIGGVICRM ACKDSRKKYG TKVIYTAHGF HFFEGASKKA WAVFYPVEKF CSHYTDVLIT | 780 |
| INREDYRNAQ KFHAKKVQYV PGIGIHTDEF RSVEINRSSK RSEFGFDDDD FVFMSTGQIS | 840 |
| VRKNHEVVIR ALAKIEKANV KYLIVGFGEL EHQLKKLVVE LGLNSRVVFA GYRDDVKELL | 900 |
| HTVDAYAFPS LQEGLPASLM EAMSVGLPVI CSRIRGNVDL IENGKGGFIY DCHDVNGFAE | 960 |
| GMNKIIGGAG RNMGATNIET MKKFDISAVN EIMERIYKNA IV | 1002 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam02397 | Bac_transf | 3.40e-75 | 91 | 262 | 1 | 173 | Bacterial sugar transferase. This Pfam family represents a conserved region from a number of different bacterial sugar transferases, involved in diverse biosynthesis pathways. |
| COG2148 | WcaJ | 2.85e-72 | 86 | 274 | 36 | 226 | Sugar transferase involved in LPS biosynthesis (colanic, teichoic acid) [Cell wall/membrane/envelope biogenesis]. |
| cd05232 | UDP_G4E_4_SDR_e | 4.14e-64 | 317 | 611 | 1 | 303 | UDP-glucose 4 epimerase, subgroup 4, extended (e) SDRs. UDP-glucose 4 epimerase (aka UDP-galactose-4-epimerase), is a homodimeric extended SDR. It catalyzes the NAD-dependent conversion of UDP-galactose to UDP-glucose, the final step in Leloir galactose synthesis. This subgroup is comprised of bacterial proteins, and includes the Staphylococcus aureus capsular polysaccharide Cap5N, which may have a role in the synthesis of UDP-N-acetyl-d-fucosamine. This subgroup has the characteristic active site tetrad and NAD-binding motif of the extended SDRs. Extended SDRs are distinct from classical SDRs. In addition to the Rossmann fold (alpha/beta folding pattern with a central beta-sheet) core region typical of all SDRs, extended SDRs have a less conserved C-terminal extension of approximately 100 amino acids. Extended SDRs are a diverse collection of proteins, and include isomerases, epimerases, oxidoreductases, and lyases; they typically have a TGXXGXXG cofactor binding motif. SDRs are a functionally diverse family of oxidoreductases that have a single domain with a structurally conserved Rossmann fold, an NAD(P)(H)-binding region, and a structurally diverse C-terminal region. Sequence identity between different SDR enzymes is typically in the 15-30% range; they catalyze a wide range of activities including the metabolism of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, and act in redox sensing. Classical SDRs have an TGXXX[AG]XG cofactor binding motif and a YXXXK active site motif, with the Tyr residue of the active site motif serving as a critical catalytic residue (Tyr-151, human 15-hydroxyprostaglandin dehydrogenase numbering). In addition to the Tyr and Lys, there is often an upstream Ser and/or an Asn, contributing to the active site; while substrate binding is in the C-terminal region, which determines specificity. The standard reaction mechanism is a 4-pro-S hydride transfer and proton relay involving the conserved Tyr and Lys, a water molecule stabilized by Asn, and nicotinamide. Atypical SDRs generally lack the catalytic residues characteristic of the SDRs, and their glycine-rich NAD(P)-binding motif is often different from the forms normally seen in classical or extended SDRs. Complex (multidomain) SDRs such as ketoreductase domains of fatty acid synthase have a GGXGXXG NAD(P)-binding motif and an altered active site motif (YXXXN). Fungal type ketoacyl reductases have a TGXXXGX(1-2)G NAD(P)-binding motif. |
| cd03808 | GT4_CapM-like | 9.30e-64 | 623 | 994 | 1 | 358 | capsular polysaccharide biosynthesis glycosyltransferase CapM and similar proteins. This family is most closely related to the GT4 family of glycosyltransferases. CapM in Staphylococcus aureus is required for the synthesis of type 1 capsular polysaccharides. |
| TIGR03025 | EPS_sugtrans | 9.97e-57 | 87 | 259 | 254 | 429 | exopolysaccharide biosynthesis polyprenyl glycosylphosphotransferase. Members of this family are generally found near other genes involved in the biosynthesis of a variety of exopolysaccharides. These proteins consist of two fused domains, an N-terminal hydrophobic domain of generally low conservation and a highly conserved C-terminal sugar transferase domain (pfam02397). Characterized and partially characterized members of this subfamily include Salmonella WbaP (originally RfbP), E. coli WcaJ, Methylobacillus EpsB, Xanthomonas GumD, Vibrio CpsA, Erwinia AmsG, Group B Streptococcus CpsE (originally CpsD), and Streptococcus suis Cps2E. Each of these is believed to act in transferring the sugar from, for instance, UDP-glucose or UDP-galactose, to a lipid carrier such as undecaprenyl phosphate as the first (priming) step in the synthesis of an oligosaccharide "block". This function is encoded in the C-terminal domain. The liposaccharide is believed to be subsequently transferred through a "flippase" function from the cytoplasmic to the periplasmic face of the inner membrane by the N-terminal domain. Certain closely related transferase enzymes, such as Sinorhizobium ExoY and Lactococcus EpsD, lack the N-terminal domain and are not found by this model. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUO31262.1 | 0.0 | 1 | 1001 | 1 | 1013 |
| QMW80818.1 | 1.01e-183 | 78 | 627 | 28 | 640 |
| QIB56408.1 | 1.01e-183 | 78 | 627 | 28 | 640 |
| VCV20929.1 | 2.00e-176 | 316 | 999 | 2 | 686 |
| APC41948.1 | 1.16e-144 | 623 | 997 | 3 | 378 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5W7L_A | 2.56e-31 | 84 | 284 | 4 | 200 | Structureof Campylobacter concisus PglC I57M/Q175M variant [Campylobacter concisus 13826],5W7L_B Structure of Campylobacter concisus PglC I57M/Q175M variant [Campylobacter concisus 13826] |
| 2JJM_A | 1.06e-12 | 665 | 960 | 59 | 342 | CrystalStructure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_B Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_C Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_D Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_E Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_F Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_G Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_H Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_I Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_J Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_K Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames],2JJM_L Crystal Structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. [Bacillus anthracis str. Ames] |
| 3MBO_A | 1.19e-12 | 665 | 960 | 79 | 362 | CrystalStructure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_B Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_C Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_D Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_E Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_F Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_G Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis],3MBO_H Crystal Structure of the Glycosyltransferase BaBshA bound with UDP and L-malate [Bacillus anthracis] |
| 4XSO_A | 5.30e-10 | 804 | 991 | 181 | 375 | ChainA, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSO_B Chain B, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSP_A Chain A, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSP_B Chain B, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSR_A Chain A, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSR_B Chain B, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSU_A Chain A, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418],4XSU_B Chain B, Alr3699 protein [Nostoc sp. PCC 7120 = FACHB-418] |
| 2IW1_A | 1.47e-08 | 785 | 949 | 150 | 317 | CrystalStructure of WaaG, a glycosyltransferase involved in lipopolysaccharide biosynthesis [Escherichia coli str. K-12 substr. W3110] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P71053 | 5.41e-56 | 684 | 997 | 50 | 368 | Putative glycosyltransferase EpsD OS=Bacillus subtilis (strain 168) OX=224308 GN=epsD PE=2 SV=1 |
| Q44576 | 5.66e-30 | 86 | 259 | 333 | 516 | Undecaprenyl-phosphate glucose phosphotransferase OS=Komagataeibacter xylinus OX=28448 GN=aceA PE=3 SV=1 |
| P71241 | 7.94e-30 | 87 | 259 | 270 | 448 | UDP-glucose:undecaprenyl-phosphate glucose-1-phosphate transferase OS=Escherichia coli (strain K12) OX=83333 GN=wcaJ PE=1 SV=2 |
| P10498 | 1.06e-29 | 87 | 265 | 2 | 190 | Exopolysaccharide production protein PSS OS=Rhizobium leguminosarum bv. phaseoli OX=385 GN=pss PE=3 SV=1 |
| Q0P9D0 | 2.31e-28 | 86 | 277 | 2 | 189 | Undecaprenyl phosphate N,N'-diacetylbacillosamine 1-phosphate transferase OS=Campylobacter jejuni subsp. jejuni serotype O:2 (strain ATCC 700819 / NCTC 11168) OX=192222 GN=pglC PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.562373 | 0.041700 | 0.392783 | 0.000282 | 0.000209 | 0.002661 |