You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000044_02168
You are here: Home > Sequence: MGYG000000044_02168
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parabacteroides merdae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides merdae | |||||||||||
| CAZyme ID | MGYG000000044_02168 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 474817; End: 476358 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 28 | 303 | 1.3e-124 | 0.99644128113879 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd08999 | GH43_ABN-like | 3.65e-89 | 28 | 304 | 1 | 284 | Glycosyl hydrolase family 43 protein such as endo-alpha-L-arabinanase. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes with alpha-L-arabinofuranosidase (ABF; EC 3.2.1.55) and endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activities. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages while the ABF enzymes cleave arabinose side chains so that the combined actions of these two enzymes reduce arabinan to L-arabinose and/or arabinooligosaccharides. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| pfam04616 | Glyco_hydro_43 | 1.36e-84 | 26 | 303 | 1 | 281 | Glycosyl hydrolases family 43. The glycosyl hydrolase family 43 contains members that are arabinanases. Arabinanases hydrolyze the alpha-1,5-linked L-arabinofuranoside backbone of plant cell wall arabinans. The structure of arabinanase Arb43A from Cellvibrio japonicus reveals a five-bladed beta-propeller fold. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08991 | GH43_HoAraf43-like | 4.09e-60 | 36 | 301 | 1 | 276 | Glycosyl hydrolase family 43 protein such as Halothermothrix orenii H 168 alpha-L-arabinofuranosidase (HoAraf43;Hore_20580). This glycosyl hydrolase family 43 (GH43) subgroup includes Halothermothrix orenii H 168 alpha-L-arabinofuranosidase (EC 3.2.1.55) (HoAraf43;Hore_20580). It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. This GH43_ HoAraf43-like subgroup includes enzymes that have been annotated as having xylan-digesting beta-xylosidase (EC 3.2.1.37) and xylanase (endo-alpha-L-arabinanase, EC 3.2.1.8) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18616 | GH43_ABN-like | 2.65e-55 | 28 | 287 | 1 | 277 | Glycosyl hydrolase family 43 such as arabinan endo-1 5-alpha-L-arabinosidase. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes with endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activity. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| COG3507 | XynB2 | 2.00e-51 | 26 | 507 | 21 | 543 | Beta-xylosidase [Carbohydrate transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT49382.1 | 0.0 | 1 | 513 | 1 | 513 |
| QUT21817.1 | 1.87e-280 | 17 | 510 | 16 | 510 |
| QUR47469.1 | 1.87e-280 | 17 | 510 | 16 | 510 |
| QRO15926.1 | 1.87e-280 | 17 | 510 | 16 | 510 |
| ABR43910.1 | 1.87e-280 | 17 | 510 | 16 | 510 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6MS3_A | 2.86e-35 | 18 | 467 | 23 | 485 | Crystalstructure of the GH43 protein BlXynB mutant (K247S) from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580],6MS3_B Crystal structure of the GH43 protein BlXynB mutant (K247S) from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580] |
| 6MS2_A | 5.32e-35 | 18 | 467 | 23 | 485 | Crystalstructure of the GH43 BlXynB protein from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580] |
| 1YRZ_A | 7.43e-30 | 28 | 507 | 7 | 520 | ChainA, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125],1YRZ_B Chain B, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125] |
| 1GYD_B | 2.44e-27 | 34 | 303 | 3 | 299 | Structureof Cellvibrio cellulosa alpha-L-arabinanase [Cellvibrio japonicus] |
| 5JOW_A | 3.74e-27 | 26 | 367 | 13 | 353 | Bacteroidesovatus Xyloglucan PUL GH43A [Bacteroides ovatus ATCC 8483],5JOW_B Bacteroides ovatus Xyloglucan PUL GH43A [Bacteroides ovatus ATCC 8483],5JOX_A Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraDNJ [Bacteroides ovatus],5JOX_B Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraDNJ [Bacteroides ovatus],5JOY_A Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraLOG [Bacteroides ovatus],5JOY_B Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraLOG [Bacteroides ovatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A9ZND1 | 2.82e-30 | 24 | 369 | 3 | 364 | Xylan 1,3-beta-xylosidase OS=Vibrio sp. OX=678 GN=xloA PE=1 SV=1 |
| A7LXT8 | 2.20e-26 | 26 | 367 | 23 | 363 | Non-reducing end alpha-L-arabinofuranosidase BoGH43A OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02654 PE=1 SV=1 |
| P95470 | 3.21e-26 | 34 | 303 | 35 | 331 | Extracellular exo-alpha-(1->5)-L-arabinofuranosidase ArbA OS=Cellvibrio japonicus (strain Ueda107) OX=498211 GN=arbA PE=1 SV=1 |
| P94489 | 1.85e-25 | 27 | 339 | 4 | 338 | Beta-xylosidase OS=Bacillus subtilis (strain 168) OX=224308 GN=xynB PE=1 SV=2 |
| A7LXU0 | 1.43e-24 | 3 | 366 | 7 | 370 | Non-reducing end alpha-L-arabinofuranosidase BoGH43B OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02656 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
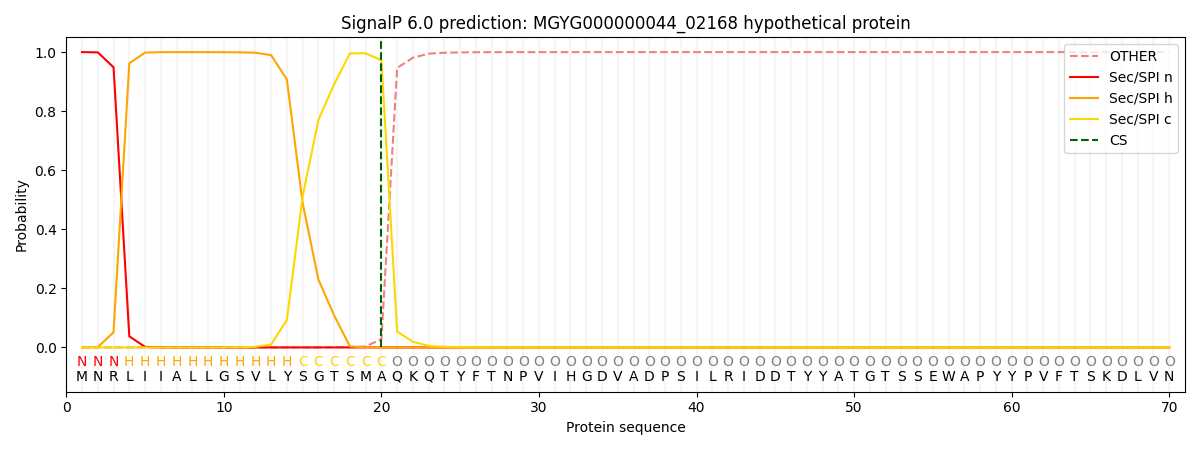
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000545 | 0.998566 | 0.000337 | 0.000174 | 0.000183 | 0.000163 |
