You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004899_00725
You are here: Home > Sequence: MGYG000004899_00725
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides sp900555635 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp900555635 | |||||||||||
| CAZyme ID | MGYG000004899_00725 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 74964; End: 77291 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH31 | 216 | 663 | 1.1e-135 | 0.9976580796252927 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam01055 | Glyco_hydro_31 | 2.35e-159 | 217 | 663 | 1 | 442 | Glycosyl hydrolases family 31. Glycosyl hydrolases are key enzymes of carbohydrate metabolism. Family 31 comprises of enzymes that are, or similar to, alpha- galactosidases. |
| cd06591 | GH31_xylosidase_XylS | 2.64e-158 | 236 | 558 | 1 | 322 | xylosidase XylS-like. XylS is a glycosyl hydrolase family 31 (GH31) alpha-xylosidase found in prokaryotes, eukaryotes, and archaea, that catalyzes the release of alpha-xylose from the non-reducing terminal side of the alpha-xyloside substrate. XylS has been characterized in Sulfolobus solfataricus where it hydrolyzes isoprimeverose, the p-nitrophenyl-beta derivative of isoprimeverose, and xyloglucan oligosaccharides, and has transxylosidic activity. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. The XylS family corresponds to subgroup 3 in the Ernst et al classification of GH31 enzymes. |
| COG1501 | YicI | 4.72e-157 | 71 | 756 | 63 | 763 | Alpha-glucosidase, glycosyl hydrolase family GH31 [Carbohydrate transport and metabolism]. |
| cd06589 | GH31 | 5.59e-66 | 236 | 537 | 1 | 248 | glycosyl hydrolase family 31 (GH31). GH31 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-xylosidase, 6-alpha-glucosyltransferase, 3-alpha-isomaltosyltransferase and alpha-1,4-glucan lyase. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. In most cases, the pyranose moiety recognized in subsite -1 of the substrate binding site is an alpha-D-glucose, though some GH31 family members show a preference for alpha-D-xylose. Several GH31 enzymes can accommodate both glucose and xylose and different levels of discrimination between the two have been observed. Most characterized GH31 enzymes are alpha-glucosidases. In mammals, GH31 members with alpha-glucosidase activity are implicated in at least three distinct biological processes. The lysosomal acid alpha-glucosidase (GAA) is essential for glycogen degradation and a deficiency or malfunction of this enzyme causes glycogen storage disease II, also known as Pompe disease. In the endoplasmic reticulum, alpha-glucosidase II catalyzes the second step in the N-linked oligosaccharide processing pathway that constitutes part of the quality control system for glycoprotein folding and maturation. The intestinal enzymes sucrase-isomaltase (SI) and maltase-glucoamylase (MGAM) play key roles in the final stage of carbohydrate digestion, making alpha-glucosidase inhibitors useful in the treatment of type 2 diabetes. GH31 alpha-glycosidases are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd06603 | GH31_GANC_GANAB_alpha | 1.27e-64 | 236 | 697 | 1 | 463 | neutral alpha-glucosidase C, neutral alpha-glucosidase AB. This subgroup includes the closely related glycosyl hydrolase family 31 (GH31) isozymes, neutral alpha-glucosidase C (GANC) and the alpha subunit of heterodimeric neutral alpha-glucosidase AB (GANAB). Initially distinguished on the basis of differences in electrophoretic mobility in starch gel, GANC and GANAB have been shown to have other differences, including those of substrate specificity. GANC and GANAB are key enzymes in glycogen metabolism that hydrolyze terminal, non-reducing 1,4-linked alpha-D-glucose residues from glycogen in the endoplasmic reticulum. The GANC/GANAB family includes the alpha-glucosidase II (ModA) from Dictyostelium discoideum as well as the alpha-glucosidase II (GLS2, or ROT2 - Reversal of TOR2 lethality protein 2) from Saccharomyces cerevisiae. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT89550.1 | 0.0 | 1 | 774 | 1 | 773 |
| QDO67868.1 | 0.0 | 1 | 774 | 1 | 773 |
| QXU42202.1 | 5.99e-312 | 2 | 774 | 4 | 776 |
| QDW23455.1 | 1.71e-311 | 15 | 772 | 17 | 774 |
| AUC19787.1 | 1.15e-310 | 18 | 774 | 23 | 809 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5JOU_A | 3.40e-154 | 16 | 774 | 21 | 955 | Bacteroidesovatus Xyloglucan PUL GH31 [Bacteroides ovatus],5JOV_A Bacteroides ovatus Xyloglucan PUL GH31 with bound 5FIdoF [Bacteroides ovatus] |
| 7KMP_A | 2.67e-141 | 19 | 772 | 57 | 975 | ChainA, Alpha-xylosidase [Xanthomonas citri pv. citri str. 306],7KNC_A Chain A, Alpha-xylosidase [Xanthomonas citri pv. citri str. 306] |
| 2XVG_A | 1.34e-137 | 22 | 772 | 49 | 984 | crystalstructure of alpha-xylosidase (GH31) from Cellvibrio japonicus [Cellvibrio japonicus],2XVK_A crystal structure of alpha-xylosidase (GH31) from Cellvibrio japonicus in complex with 5-fluoro-alpha-D-xylopyranosyl fluoride [Cellvibrio japonicus],2XVL_A crystal structure of alpha-xylosidase (GH31) from Cellvibrio japonicus in complex with Pentaerythritol propoxylate (5 4 PO OH) [Cellvibrio japonicus] |
| 4B9Y_A | 1.27e-69 | 28 | 761 | 47 | 779 | CrystalStructure of Apo Agd31B, alpha-transglucosylase in Glycoside Hydrolase Family 31 [Cellvibrio japonicus],4B9Z_A Crystal Structure of Agd31B, alpha-transglucosylase, complexed with Acarbose [Cellvibrio japonicus],4BA0_A Crystal Structure of Agd31B, alpha-transglucosylase, complexed with 5F-alpha-GlcF [Cellvibrio japonicus] |
| 5I23_A | 1.67e-69 | 28 | 761 | 24 | 756 | CrystalStructure of Agd31B, alpha-transglucosylase in Glycoside Hydrolase Family 31, in complex with Cyclophellitol Aziridine probe CF022 [Cellvibrio japonicus Ueda107],5I24_A Crystal Structure of Agd31B, alpha-transglucosylase in Glycoside Hydrolase Family 31, in complex with Cyclophellitol Aziridine probe CF021 [Cellvibrio japonicus Ueda107],5NPB_A Crystal Structure of cjAgd31B (alpha-transglucosylase from Glycoside Hydrolase Family 31) in complex with alpha Cyclophellitol Cyclosulfate probe ME647 [Cellvibrio japonicus],5NPE_A Crystal Structure of cjAgd31B (alpha-transglucosylase from Glycoside Hydrolase Family 31) in complex with beta Cyclophellitol Aziridine probe KY358 [Cellvibrio japonicus Ueda107] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9P999 | 4.66e-176 | 31 | 769 | 1 | 724 | Alpha-xylosidase OS=Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) OX=273057 GN=xylS PE=1 SV=1 |
| A7LXT0 | 5.04e-153 | 23 | 774 | 27 | 954 | Alpha-xylosidase BoGH31A OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02646 PE=1 SV=1 |
| Q9F234 | 5.47e-86 | 21 | 726 | 30 | 736 | Alpha-glucosidase 2 OS=Bacillus thermoamyloliquefaciens OX=1425 PE=3 SV=1 |
| B3PEE6 | 6.85e-69 | 28 | 761 | 47 | 779 | Oligosaccharide 4-alpha-D-glucosyltransferase OS=Cellvibrio japonicus (strain Ueda107) OX=498211 GN=agd31B PE=1 SV=1 |
| A2QTU5 | 1.39e-64 | 138 | 656 | 158 | 722 | Alpha-xylosidase A OS=Aspergillus niger (strain CBS 513.88 / FGSC A1513) OX=425011 GN=axlA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
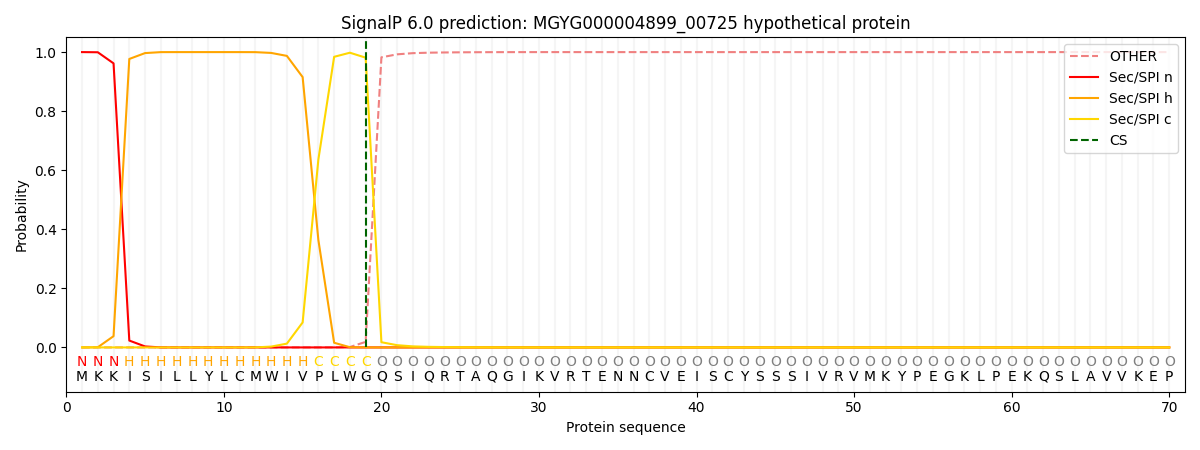
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000209 | 0.999215 | 0.000153 | 0.000141 | 0.000132 | 0.000128 |
