You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004510_00542
You are here: Home > Sequence: MGYG000004510_00542
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp002481295 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp002481295 | |||||||||||
| CAZyme ID | MGYG000004510_00542 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 43755; End: 45155 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 25 | 276 | 2.2e-123 | 0.9963503649635036 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd08998 | GH43_Arb43a-like | 4.41e-72 | 26 | 270 | 1 | 277 | Glycosyl hydrolase family 43 protein such as Bacillus subtilis subsp. subtilis str. 168 endo-alpha-1,5-L-arabinanase Arb43A. This glycosyl hydrolase family 43 (GH43) subgroup belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages while the ABF enzymes cleave arabinose side chains so that the combined actions of these two enzymes reduce arabinan to L-arabinose and/or arabinooligosaccharides. Many of these enzymes such as the Bacillus subtilis arabinanase Abn2, that hydrolyzes sugar beet arabinan (branched), linear alpha-1,5-L-arabinan and pectin, are different from other arabinases; they are organized into two different domains with a divalent metal cluster close to the catalytic residues to guarantee the correct protonation state of the catalytic residues and consequently the enzyme activity. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18830 | GH43_CjArb43A-like | 9.51e-41 | 26 | 270 | 1 | 290 | Glycosyl hydrolase family 43 protein such as Cellvibrio japonicus Ueda107 endo-alpha-1,5-L-arabinanase / exo-alpha-1,5-L-arabinanase 43A (ArbA;CJA_0805) (Arb43A). This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes annotated with alpha-L-arabinofuranosidase (ABF; EC 3.2.1.55) and endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activities, and includes the bifunctional Cellvibrio japonicus Ueda107 endo-alpha-1,5-L-arabinanase / exo-alpha-1,5-L-arabinanase 43A (ArbA;CJA_0805) (Arb43A). It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages while the ABF enzymes cleave arabinose side chains so that the combined actions of these two enzymes reduce arabinan to L-arabinose and/or arabinooligosaccharides. Many of these enzymes such as the Bacillus subtilis arabinanase Abn2, that hydrolyzes sugar beet arabinan (branched), linear alpha-1,5-L-arabinan and pectin, are different from other arabinases; they are organized into two different domains with a divalent metal cluster close to the catalytic residues to guarantee the correct protonation state of the catalytic residues and consequently the enzyme activity. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18829 | GH43_BsArb43A-like | 2.91e-36 | 102 | 270 | 106 | 272 | Glycosyl hydrolase family 43 protein such as Bacillus subtilis subsp. subtilis str. 168 endo-alpha-1,5-L-arabinanase Arb43A. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes annotated as having endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activities, and includes Bacillus subtilis subsp. subtilis str. 168 endo-alpha-1,5-L-arabinanase (AbnA;BSU28810) (Arb43A). It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages while the arabinofuranosidase (ABF; EC 3.2.1.55) enzymes cleave arabinose side chains so that the combined actions of these two enzymes reduce arabinan to L-arabinose and/or arabinooligosaccharides. Many of these enzymes such as the Bacillus subtilis arabinanase Abn2, that hydrolyzes sugar beet arabinan (branched), linear alpha-1,5-L-arabinan and pectin, are different from other arabinases; they are organized into two different domains with a divalent metal cluster close to the catalytic residues to guarantee the correct protonation state of the catalytic residues and consequently the enzyme activity. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08988 | GH43_ABN | 1.92e-35 | 50 | 270 | 62 | 277 | Glycosyl hydrolase family 43. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes with alpha-L-arabinofuranosidase (ABF; EC 3.2.1.55) and endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activities. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages while the ABF enzymes cleave arabinose side chains so that the combined actions of these two enzymes reduce arabinan to L-arabinose and/or arabinooligosaccharides. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18616 | GH43_ABN-like | 3.71e-35 | 50 | 243 | 73 | 259 | Glycosyl hydrolase family 43 such as arabinan endo-1 5-alpha-L-arabinosidase. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes with endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activity. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADE82026.1 | 1.88e-251 | 1 | 461 | 1 | 481 |
| QVJ79577.1 | 3.11e-250 | 1 | 461 | 1 | 481 |
| QNO39518.1 | 1.95e-240 | 6 | 460 | 7 | 483 |
| BCS85396.1 | 2.49e-240 | 7 | 461 | 11 | 484 |
| ADI70676.1 | 1.61e-233 | 10 | 460 | 11 | 479 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1WL7_A | 4.13e-34 | 50 | 275 | 84 | 310 | Structureof the thermostable arabinanase [Geobacillus thermodenitrificans] |
| 6A8H_A | 5.01e-34 | 50 | 275 | 85 | 311 | Crystalstructure of endo-arabinanase ABN-TS D27A mutant in complex with arabinotriose [Geobacillus thermodenitrificans] |
| 6A8I_A | 2.53e-33 | 50 | 275 | 85 | 311 | Crystalstructure of endo-arabinanase ABN-TS D147N mutant in complex with arabinohexaose [Geobacillus thermodenitrificans],6A8I_B Crystal structure of endo-arabinanase ABN-TS D147N mutant in complex with arabinohexaose [Geobacillus thermodenitrificans] |
| 3CU9_A | 3.01e-33 | 50 | 275 | 84 | 310 | Highresolution crystal structure of 1,5-alpha-L-arabinanase from Geobacillus Stearothermophilus [Geobacillus stearothermophilus] |
| 3D60_A | 3.01e-33 | 50 | 275 | 84 | 310 | ChainA, Intracellular arabinanase [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q93HT9 | 2.31e-33 | 50 | 275 | 85 | 311 | Intracellular endo-alpha-(1->5)-L-arabinanase OS=Geobacillus thermodenitrificans OX=33940 GN=abn-ts PE=1 SV=1 |
| B3EYM8 | 1.69e-32 | 50 | 275 | 85 | 311 | Intracellular endo-alpha-(1->5)-L-arabinanase OS=Geobacillus stearothermophilus OX=1422 GN=abnB PE=1 SV=1 |
| P95470 | 2.04e-26 | 50 | 275 | 95 | 331 | Extracellular exo-alpha-(1->5)-L-arabinofuranosidase ArbA OS=Cellvibrio japonicus (strain Ueda107) OX=498211 GN=arbA PE=1 SV=1 |
| P94522 | 3.47e-26 | 102 | 275 | 150 | 321 | Extracellular endo-alpha-(1->5)-L-arabinanase 1 OS=Bacillus subtilis (strain 168) OX=224308 GN=abnA PE=1 SV=3 |
| Q5B8T6 | 6.57e-14 | 112 | 285 | 166 | 359 | Probable arabinan endo-1,5-alpha-L-arabinosidase D OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=abnD PE=2 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
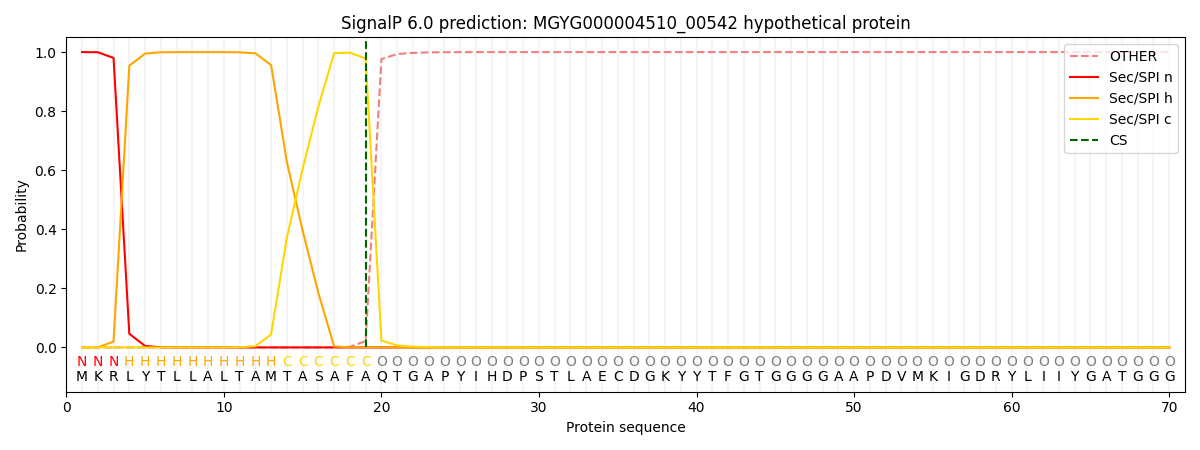
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000222 | 0.999087 | 0.000167 | 0.000180 | 0.000160 | 0.000148 |
