You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003878_00155
You are here: Home > Sequence: MGYG000003878_00155
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
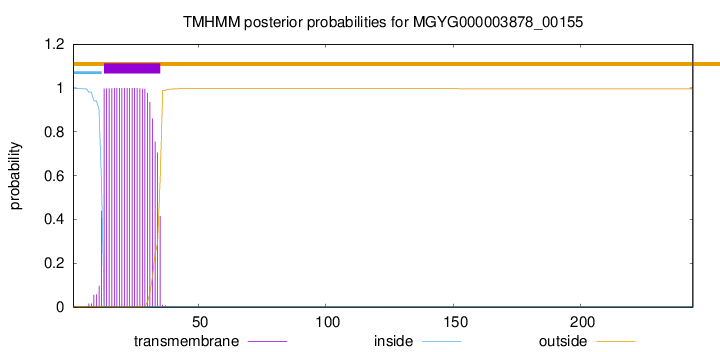
TMHMM annotations
Basic Information help
| Species | Prevotella oris | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella oris | |||||||||||
| CAZyme ID | MGYG000003878_00155 | |||||||||||
| CAZy Family | GT51 | |||||||||||
| CAZyme Description | Biosynthetic peptidoglycan transglycosylase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 18698; End: 19432 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT51 | 55 | 219 | 2e-52 | 0.9265536723163842 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| PRK00056 | mtgA | 9.18e-116 | 5 | 228 | 8 | 234 | monofunctional biosynthetic peptidoglycan transglycosylase; Provisional |
| TIGR02070 | mono_pep_trsgly | 2.21e-86 | 6 | 220 | 2 | 219 | monofunctional biosynthetic peptidoglycan transglycosylase. This family is one of the transglycosylases involved in the late stages of peptidoglycan biosynthesis. Members tend to be small, about 240 amino acids in length, and consist almost entirely of a domain described by pfam00912 for transglycosylases. Species with this protein will have several other transglycosylases as well. All species with this protein are Proteobacteria that produce murein (peptidoglycan). [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan] |
| pfam00912 | Transgly | 1.32e-68 | 55 | 220 | 12 | 177 | Transglycosylase. The penicillin-binding proteins are bifunctional proteins consisting of transglycosylase and transpeptidase in the N- and C-terminus respectively. The transglycosylase domain catalyzes the polymerization of murein glycan chains. |
| COG0744 | MrcB | 3.09e-66 | 1 | 220 | 8 | 241 | Membrane carboxypeptidase (penicillin-binding protein) [Cell wall/membrane/envelope biogenesis]. |
| TIGR02074 | PBP_1a_fam | 1.92e-47 | 58 | 220 | 4 | 166 | penicillin-binding protein, 1A family. Bacterial that synthesize a cell wall of peptidoglycan (murein) generally have several transglycosylases and transpeptidases for the task. This family consists of bifunctional transglycosylase/transpeptidase penicillin-binding proteins (PBP). In the Proteobacteria, this family includes PBP 1A but not the paralogous PBP 1B (TIGR02071). This family also includes related proteins, often designated PBP 1A, from other bacterial lineages. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan] |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| VEH15611.1 | 6.93e-182 | 1 | 244 | 1 | 244 |
| ALO48142.1 | 5.20e-136 | 7 | 242 | 6 | 240 |
| BCS84223.1 | 6.04e-131 | 38 | 243 | 1 | 206 |
| QUB46829.1 | 2.60e-129 | 7 | 231 | 6 | 230 |
| AGB29041.1 | 5.89e-128 | 9 | 242 | 2 | 232 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3NB6_A | 8.67e-28 | 58 | 219 | 22 | 183 | Crystalstructure of Aquifex aeolicus peptidoglycan glycosyltransferase in complex with Methylphosphoryl Neryl Moenomycin [Aquifex aeolicus] |
| 2OQO_A | 1.22e-27 | 58 | 219 | 22 | 183 | Crystalstructure of a peptidoglycan glycosyltransferase from a class A PBP: insight into bacterial cell wall synthesis [Aquifex aeolicus VF5],3D3H_A Crystal structure of a complex of the peptidoglycan glycosyltransferase domain from Aquifex aeolicus and neryl moenomycin A [Aquifex aeolicus],3NB7_A Crystal structure of Aquifex Aeolicus Peptidoglycan Glycosyltransferase in complex with Decarboxylated Neryl Moenomycin [Aquifex aeolicus] |
| 4OON_A | 8.55e-24 | 71 | 219 | 54 | 202 | Crystalstructure of PBP1a in complex with compound 17 ((4Z,8S,11E,14S)-5-(2-amino-1,3-thiazol-4-yl)-14-(5,6-dihydroxy-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-8-formyl-2-methyl-6-oxo-3,10-dioxa-4,7,11-triazatetradeca-4,11-diene-2,12,14-tricarboxylic acid) [Pseudomonas aeruginosa PAO1] |
| 3DWK_A | 4.74e-21 | 44 | 200 | 19 | 171 | ChainA, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_B Chain B, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_C Chain C, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_D Chain D, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL] |
| 2OLU_A | 1.22e-20 | 44 | 200 | 28 | 180 | StructuralInsight Into the Transglycosylation Step Of Bacterial Cell Wall Biosynthesis : Apoenzyme [Staphylococcus aureus],2OLV_A Structural Insight Into the Transglycosylation Step Of Bacterial Cell Wall Biosynthesis : Donor Ligand Complex [Staphylococcus aureus],2OLV_B Structural Insight Into the Transglycosylation Step Of Bacterial Cell Wall Biosynthesis : Donor Ligand Complex [Staphylococcus aureus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8A8H2 | 2.06e-90 | 7 | 226 | 9 | 230 | Biosynthetic peptidoglycan transglycosylase OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=mtgA PE=3 SV=1 |
| Q3V815 | 1.68e-89 | 7 | 226 | 8 | 226 | Biosynthetic peptidoglycan transglycosylase OS=Bacteroides fragilis (strain YCH46) OX=295405 GN=mtgA PE=3 SV=1 |
| Q3V7G2 | 1.68e-89 | 7 | 226 | 8 | 226 | Biosynthetic peptidoglycan transglycosylase OS=Bacteroides fragilis (strain ATCC 25285 / DSM 2151 / CCUG 4856 / JCM 11019 / NCTC 9343 / Onslow) OX=272559 GN=mtgA PE=3 SV=1 |
| Q3V7R3 | 3.15e-70 | 15 | 230 | 18 | 230 | Biosynthetic peptidoglycan transglycosylase OS=Bdellovibrio bacteriovorus (strain ATCC 15356 / DSM 50701 / NCIMB 9529 / HD100) OX=264462 GN=mtgA PE=3 SV=1 |
| Q1IGD6 | 1.60e-64 | 16 | 219 | 16 | 218 | Biosynthetic peptidoglycan transglycosylase OS=Pseudomonas entomophila (strain L48) OX=384676 GN=mtgA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000038 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |