You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003681_00227
You are here: Home > Sequence: MGYG000003681_00227
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides stercoris | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides stercoris | |||||||||||
| CAZyme ID | MGYG000003681_00227 | |||||||||||
| CAZy Family | CE7 | |||||||||||
| CAZyme Description | Acetyl esterase Axe7A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 288994; End: 290313 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE7 | 131 | 433 | 1.8e-90 | 0.9584664536741214 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3458 | Axe1 | 6.85e-49 | 130 | 420 | 9 | 299 | Cephalosporin-C deacetylase or related acetyl esterase [Secondary metabolites biosynthesis, transport and catabolism]. |
| pfam05448 | AXE1 | 2.47e-47 | 130 | 420 | 8 | 299 | Acetyl xylan esterase (AXE1). This family consists of several bacterial acetyl xylan esterase proteins. Acetyl xylan esterases are enzymes that hydrolyze the ester linkages of the acetyl groups in position 2 and/or 3 of the xylose moieties of natural acetylated xylan from hardwood. These enzymes are one of the accessory enzymes which are part of the xylanolytic system, together with xylanases, beta-xylosidases, alpha-arabinofuranosidases and methylglucuronidases; these are all required for the complete hydrolysis of xylan. |
| COG1506 | DAP2 | 1.37e-10 | 184 | 439 | 373 | 618 | Dipeptidyl aminopeptidase/acylaminoacyl peptidase [Amino acid transport and metabolism]. |
| COG0412 | DLH | 5.81e-04 | 286 | 400 | 97 | 214 | Dienelactone hydrolase [Secondary metabolites biosynthesis, transport and catabolism]. |
| COG4287 | PqaA | 0.003 | 295 | 431 | 228 | 380 | PhoPQ-activated pathogenicity-related protein [General function prediction only]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QDO69181.1 | 2.96e-274 | 23 | 439 | 22 | 438 |
| QIU97381.1 | 2.94e-271 | 5 | 439 | 2 | 435 |
| QNL39512.1 | 2.94e-271 | 19 | 439 | 16 | 435 |
| QUT93205.1 | 5.45e-270 | 1 | 439 | 1 | 438 |
| ALJ61266.1 | 5.45e-270 | 1 | 439 | 1 | 438 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5GMA_A | 1.40e-29 | 131 | 420 | 25 | 315 | Crystalstructure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_B Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_C Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_D Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_E Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_F Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8] |
| 3M81_A | 1.93e-29 | 131 | 420 | 25 | 315 | Crystalstructure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],3M81_B Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],3M81_C Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],3M81_D Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],3M81_E Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],3M81_F Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.50 A resolution (native apo structure) [Thermotoga maritima],5FDF_A Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5FDF_B Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5FDF_C Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5FDF_D Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5FDF_E Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5FDF_F Crystal structure of the monoclinic form of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 1.76 Angstrom resolution [Thermotoga maritima],5JIB_A Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8],5JIB_B Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8],5JIB_C Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8],5JIB_D Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8],5JIB_E Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8],5JIB_F Crystal structure of the Thermotoga maritima acetyl esterase (TM0077) complex with a substrate analog [Thermotoga maritima MSB8] |
| 3M82_A | 9.51e-29 | 131 | 420 | 25 | 315 | Crystalstructure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M82_B Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M82_C Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M82_D Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M82_E Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M82_F Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.40 A resolution (PMSF inhibitor complex structure) [Thermotoga maritima],3M83_A Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima],3M83_B Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima],3M83_C Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima],3M83_D Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima],3M83_E Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima],3M83_F Crystal structure of Acetyl xylan esterase (TM0077) from THERMOTOGA MARITIMA at 2.12 A resolution (paraoxon inhibitor complex structure) [Thermotoga maritima] |
| 1VLQ_A | 6.42e-28 | 131 | 420 | 25 | 315 | Crystalstructure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_B Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_C Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_D Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_E Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_F Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_G Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_H Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_I Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_J Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_K Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8],1VLQ_L Crystal structure of Acetyl xylan esterase (TM0077) from Thermotoga maritima at 2.10 A resolution [Thermotoga maritima MSB8] |
| 1ODS_A | 3.09e-27 | 130 | 439 | 9 | 317 | CephalosporinC deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_B Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_C Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_D Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_E Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_F Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_G Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_H Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| D5EXI2 | 5.94e-210 | 3 | 439 | 11 | 439 | Acetyl esterase Axe7A OS=Prevotella ruminicola (strain ATCC 19189 / JCM 8958 / 23) OX=264731 GN=axe7A PE=1 SV=1 |
| Q9WXT2 | 8.44e-29 | 131 | 420 | 13 | 303 | Cephalosporin-C deacetylase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=axeA PE=1 SV=1 |
| P94388 | 1.23e-26 | 130 | 439 | 9 | 317 | Cephalosporin-C deacetylase OS=Bacillus subtilis (strain 168) OX=224308 GN=cah PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
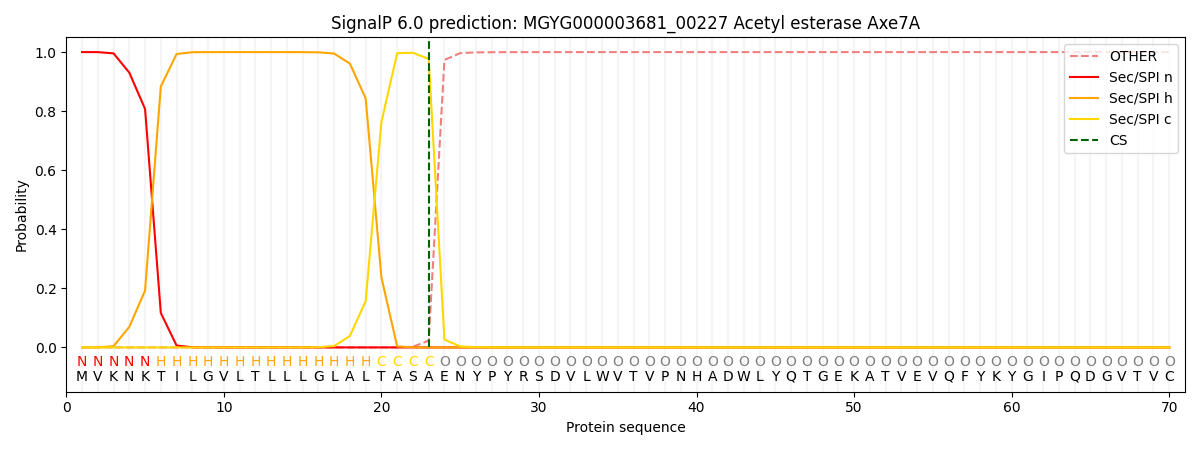
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000287 | 0.998987 | 0.000175 | 0.000203 | 0.000172 | 0.000157 |
