You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003424_02149
You are here: Home > Sequence: MGYG000003424_02149
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | SFTJ01 sp004557385 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; SFTJ01; SFTJ01 sp004557385 | |||||||||||
| CAZyme ID | MGYG000003424_02149 | |||||||||||
| CAZy Family | GH20 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 6677; End: 8509 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH20 | 103 | 384 | 7.4e-58 | 0.9673590504451038 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd02742 | GH20_hexosaminidase | 4.13e-66 | 109 | 383 | 1 | 302 | Beta-N-acetylhexosaminidases of glycosyl hydrolase family 20 (GH20) catalyze the removal of beta-1,4-linked N-acetyl-D-hexosamine residues from the non-reducing ends of N-acetyl-beta-D-hexosaminides including N-acetylglucosides and N-acetylgalactosides. These enzymes are broadly distributed in microorganisms, plants and animals, and play roles in various key physiological and pathological processes. These processes include cell structural integrity, energy storage, cellular signaling, fertilization, pathogen defense, viral penetration, the development of carcinomas, inflammatory events and lysosomal storage disorders. The GH20 enzymes include the eukaryotic beta-N-acetylhexosaminidases A and B, the bacterial chitobiases, dispersin B, and lacto-N-biosidase. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by the solvent or the enzyme, but by the substrate itself. |
| pfam00728 | Glyco_hydro_20 | 3.85e-52 | 107 | 385 | 1 | 345 | Glycosyl hydrolase family 20, catalytic domain. This domain has a TIM barrel fold. |
| cd06563 | GH20_chitobiase-like | 2.64e-46 | 107 | 321 | 1 | 270 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This GH20 domain family includes an N-acetylglucosamidase (GlcNAcase A) from Pseudoalteromonas piscicida and an N-acetylhexosaminidase (SpHex) from Streptomyces plicatus. SpHex lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| cd06562 | GH20_HexA_HexB-like | 3.44e-45 | 107 | 427 | 1 | 348 | Beta-N-acetylhexosaminidases catalyze the removal of beta-1,4-linked N-acetyl-D-hexosamine residues from the non-reducing ends of N-acetyl-beta-D-hexosaminides including N-acetylglucosides and N-acetylgalactosides. The hexA and hexB genes encode the alpha- and beta-subunits of the two major beta-N-acetylhexosaminidase isoenzymes, N-acetyl-beta-D-hexosaminidase A (HexA) and beta-N-acetylhexosaminidase B (HexB). Both the alpha and the beta catalytic subunits have a TIM-barrel fold and belong to the glycosyl hydrolase family 20 (GH20). The HexA enzyme is a heterodimer containing one alpha and one beta subunit while the HexB enzyme is a homodimer containing two beta-subunits. Hexosaminidase mutations cause an inability to properly hydrolyze certain sphingolipids which accumulate in lysosomes within the brain, resulting in the lipid storage disorders Tay-Sachs and Sandhoff. Mutations in the alpha subunit cause in a deficiency in the HexA enzyme and result in Tay-Sachs, mutations in the beta-subunit cause in a deficiency in both HexA and HexB enzymes and result in Sandhoff disease. In both disorders GM(2) gangliosides accumulate in lysosomes. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| cd06570 | GH20_chitobiase-like_1 | 2.28e-37 | 107 | 383 | 1 | 296 | A functionally uncharacterized subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the chitobiase of Serratia marcescens, a beta-N-1,4-acetylhexosaminidase that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This subgroup lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CDN32025.1 | 1.77e-216 | 1 | 609 | 1 | 604 |
| QUB47710.1 | 4.59e-185 | 6 | 608 | 16 | 621 |
| ANU62653.1 | 4.53e-170 | 1 | 595 | 1 | 606 |
| ASB36858.1 | 4.53e-170 | 1 | 595 | 1 | 606 |
| QQR10010.1 | 4.53e-170 | 1 | 595 | 1 | 606 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7DUP_A | 3.49e-28 | 39 | 251 | 55 | 316 | ChainA, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVA_A Chain A, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVA_B Chain B, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron] |
| 6EZR_A | 1.19e-27 | 63 | 252 | 217 | 439 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZR_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZS_A Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6EZS_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6K35_A Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi],6K35_B Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi] |
| 7DVB_A | 1.51e-27 | 39 | 251 | 55 | 316 | ChainA, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_B Chain B, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_C Chain C, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron],7DVB_D Chain D, Beta-N-acetylhexosaminidase [Bacteroides thetaiotaomicron] |
| 6EZT_A | 1.17e-26 | 63 | 252 | 214 | 436 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi],6EZT_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi] |
| 3GH4_A | 1.47e-26 | 60 | 313 | 120 | 413 | Crystalstructure of beta-hexosaminidase from Paenibacillus sp. TS12 [Paenibacillus sp.],3GH5_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with GlcNAc [Paenibacillus sp.],3GH7_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with GalNAc [Paenibacillus sp.],3SUR_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with NAG-thiazoline. [Paenibacillus sp. TS12],3SUS_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with Gal-NAG-thiazoline [Paenibacillus sp. TS12],3SUT_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with PUGNAc [Paenibacillus sp. TS12],3SUU_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with Gal-PUGNAc [Paenibacillus sp. TS12],3SUV_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with NHAc-DNJ [Paenibacillus sp. TS12],3SUW_A Crystal structure of beta-hexosaminidase from Paenibacillus sp. TS12 in complex with NHAc-CAS [Paenibacillus sp. TS12] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P96155 | 2.19e-29 | 82 | 252 | 232 | 436 | Beta-hexosaminidase OS=Vibrio furnissii OX=29494 GN=exoI PE=1 SV=1 |
| P49008 | 1.58e-26 | 53 | 255 | 102 | 339 | Beta-hexosaminidase OS=Porphyromonas gingivalis (strain ATCC BAA-308 / W83) OX=242619 GN=nahA PE=3 SV=2 |
| E9DFH0 | 3.85e-26 | 59 | 208 | 118 | 273 | Beta-hexosaminidase 1 OS=Coccidioides posadasii (strain RMSCC 757 / Silveira) OX=443226 GN=HEX1 PE=1 SV=1 |
| Q9HGI3 | 7.13e-26 | 59 | 208 | 125 | 281 | Beta-hexosaminidase OS=Emericella nidulans OX=162425 GN=nagA PE=1 SV=1 |
| Q7WUL4 | 8.57e-26 | 58 | 298 | 78 | 350 | Beta-N-acetylhexosaminidase OS=Cellulomonas fimi OX=1708 GN=hex20 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
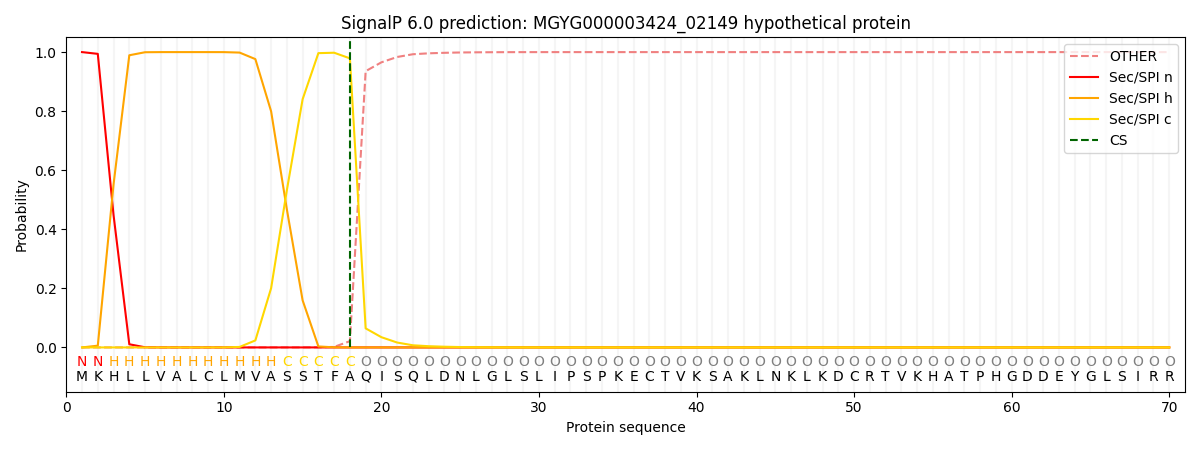
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000258 | 0.999134 | 0.000163 | 0.000151 | 0.000144 | 0.000140 |
