You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003363_01963
You are here: Home > Sequence: MGYG000003363_01963
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
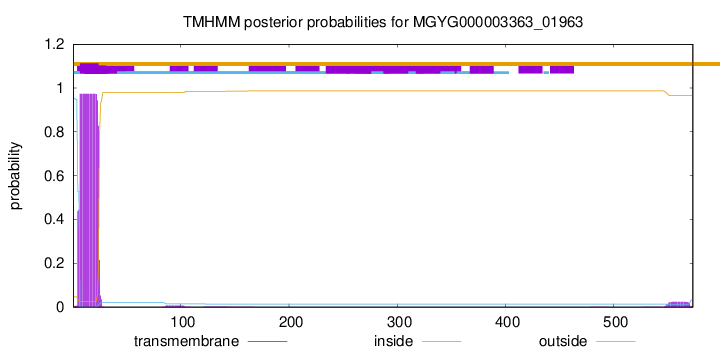
TMHMM annotations
Basic Information help
| Species | Bacteroides sp900766195 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp900766195 | |||||||||||
| CAZyme ID | MGYG000003363_01963 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | Non-reducing end alpha-L-arabinofuranosidase BoGH43A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 109109; End: 110830 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 67 | 360 | 1.2e-114 | 0.9928825622775801 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18617 | GH43_XynB-like | 2.43e-166 | 67 | 360 | 1 | 284 | Glycosyl hydrolase family 43, such as Bacteroides ovatus alpha-L-arabinofuranosidase (BoGH43, XynB). This glycosyl hydrolase family 43 (GH43) subgroup includes enzymes that have been characterized to have alpha-L-arabinofuranosidase (EC 3.2.1.55) and beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activities. Beta-1,4-xylosidases are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Also included in this subfamily are Bacteroides ovatus alpha-L-arabinofuranosidases, BoGH43A and BoGH43B, both having a two-domain architecture, consisting of an N-terminal 5-bladed beta-propeller domain harboring the catalytic active site, and a C-terminal beta-sandwich domain. However, despite significant functional overlap between these two enzymes, BoGH43A and BoGH43B share just 41% sequence identity. The latter appears to be significantly less active on the same substrates, suggesting that these paralogs may play subtly different roles during the degradation of xyloglucans from different sources, or may function most optimally at different stages in the catabolism of xyloglucan oligosaccharides (XyGOs), for example before or after hydrolysis of certain side-chain moieties. It also includes Phanerochaete chrysosporium BKM-F-1767 Xyl, a bifunctional xylosidase/arabinofuranosidase. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| COG3507 | XynB2 | 1.99e-113 | 61 | 573 | 17 | 533 | Beta-xylosidase [Carbohydrate transport and metabolism]. |
| pfam04616 | Glyco_hydro_43 | 5.51e-96 | 65 | 360 | 1 | 281 | Glycosyl hydrolases family 43. The glycosyl hydrolase family 43 contains members that are arabinanases. Arabinanases hydrolyze the alpha-1,5-linked L-arabinofuranoside backbone of plant cell wall arabinans. The structure of arabinanase Arb43A from Cellvibrio japonicus reveals a five-bladed beta-propeller fold. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd09000 | GH43_SXA-like | 2.82e-92 | 67 | 362 | 1 | 292 | Glycosyl hydrolase family 43, such as Selenomonas ruminantium beta-D-xylosidase SXA. This glycosyl hydrolase family 43 (GH43) includes enzymes that have been characterized to mainly have beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity, including Selenomonas ruminantium (Xsa;Sxa;SXA), Bifidobacterium adolescentis ATCC 15703 (XylC;XynB;BAD_0428) and Bacillus sp. KK-1 XylB. They are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. These enzymes possess an additional C-terminal beta-sandwich domain that restricts access for substrates to a portion of the active site to form a pocket. The active-site pockets comprise of two subsites, with binding capacity for two monosaccharide moieties and a single route of access for small molecules such as substrate. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18833 | GH43_PcXyl-like | 1.48e-91 | 67 | 360 | 1 | 291 | Glycosyl hydrolase family 43 protein such as the bifunctional Phanerochaete chrysosporium xylosidase/arabinofuranosidase (Xyl;PcXyl). This glycosyl hydrolase family 43 (GH43) subgroup includes Phanerochaete chrysosporium BKM-F-1767 Xyl, a characterized bifunctional enzyme with beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37)/ alpha-L-arabinofuranosidase (EC 3.2.1.55) activities. This subgroup belongs to the GH43_XybB subgroup of the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. The GH43_XybB subgroup includes enzymes having beta-1,4-xylosidase and alpha-L-arabinofuranosidase activities. Beta-1,4-xylosidases are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43_XybB subgroup includes Bacteroides ovatus alpha-L-arabinofuranosidases, BoGH43A and BoGH43B, both having a two-domain architecture, consisting of an N-terminal 5-bladed beta-propeller domain harboring the catalytic active site, and a C-terminal beta-sandwich domain. However, despite significant functional overlap between these two enzymes, BoGH43A and BoGH43B share just 41% sequence identity. The latter appears to be significantly less active on the same substrates, suggesting that these paralogs may play subtly different roles during the degradation of xyloglucans from different sources, or may function most optimally at different stages in the catabolism of xyloglucan oligosaccharides (XyGOs), for example before or after hydrolysis of certain side-chain moieties. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADD61475.1 | 3.26e-308 | 38 | 572 | 14 | 550 |
| QRQ48294.1 | 1.20e-305 | 38 | 572 | 41 | 577 |
| QJR60933.1 | 1.24e-305 | 22 | 572 | 24 | 578 |
| QJR77862.1 | 1.24e-305 | 22 | 572 | 24 | 578 |
| ALA72083.1 | 1.24e-305 | 22 | 572 | 24 | 578 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7ERL_A | 3.25e-150 | 37 | 569 | 2 | 537 | ChainA, Beta-xylanase [Bacteroides intestinalis],7ERL_B Chain B, Beta-xylanase [Bacteroides intestinalis] |
| 5Z5D_A | 1.65e-103 | 65 | 569 | 3 | 489 | Crystalstructure of a thermostable glycoside hydrolase family 43 {beta}-1,4-xylosidase from Geobacillus thermoleovorans IT-08 [Geobacillus thermoleovorans],5Z5F_A Crystal structure of a thermostable glycoside hydrolase family 43 {beta}-1,4-xylosidase from Geobacillus thermoleovorans IT-08 in complex with L-arabinose [Geobacillus thermoleovorans],5Z5H_A Crystal structure of a thermostable glycoside hydrolase family 43 {beta}-1,4-xylosidase from Geobacillus thermoleovorans IT-08 in complex with D-xylose [Geobacillus thermoleovorans],5Z5I_A Crystal structure of a thermostable glycoside hydrolase family 43 {beta}-1,4-xylosidase from Geobacillus thermoleovorans IT-08 in complex with L-arabinose and D-xylose [Geobacillus thermoleovorans] |
| 5JOW_A | 1.24e-94 | 65 | 568 | 13 | 489 | Bacteroidesovatus Xyloglucan PUL GH43A [Bacteroides ovatus ATCC 8483],5JOW_B Bacteroides ovatus Xyloglucan PUL GH43A [Bacteroides ovatus ATCC 8483],5JOX_A Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraDNJ [Bacteroides ovatus],5JOX_B Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraDNJ [Bacteroides ovatus],5JOY_A Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraLOG [Bacteroides ovatus],5JOY_B Bacteroides ovatus Xyloglucan PUL GH43A in complex with AraLOG [Bacteroides ovatus] |
| 5JOZ_A | 9.33e-94 | 64 | 569 | 4 | 485 | Bacteroidesovatus Xyloglucan PUL GH43B [Bacteroides ovatus],5JOZ_B Bacteroides ovatus Xyloglucan PUL GH43B [Bacteroides ovatus] |
| 2EXH_A | 6.18e-79 | 63 | 568 | 2 | 514 | Structureof the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_B Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_C Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_D Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P45982 | 4.96e-94 | 67 | 569 | 6 | 496 | Xylosidase/arabinosidase OS=Butyrivibrio fibrisolvens OX=831 GN=xylB PE=3 SV=1 |
| A7LXT8 | 6.37e-94 | 60 | 568 | 18 | 499 | Non-reducing end alpha-L-arabinofuranosidase BoGH43A OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02654 PE=1 SV=1 |
| A7LXU0 | 7.52e-93 | 64 | 569 | 26 | 507 | Non-reducing end alpha-L-arabinofuranosidase BoGH43B OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02656 PE=1 SV=2 |
| P07129 | 1.69e-72 | 67 | 567 | 5 | 511 | Beta-xylosidase OS=Bacillus pumilus OX=1408 GN=xynB PE=1 SV=2 |
| P77713 | 1.00e-69 | 67 | 568 | 5 | 515 | Putative beta-xylosidase OS=Escherichia coli (strain K12) OX=83333 GN=yagH PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
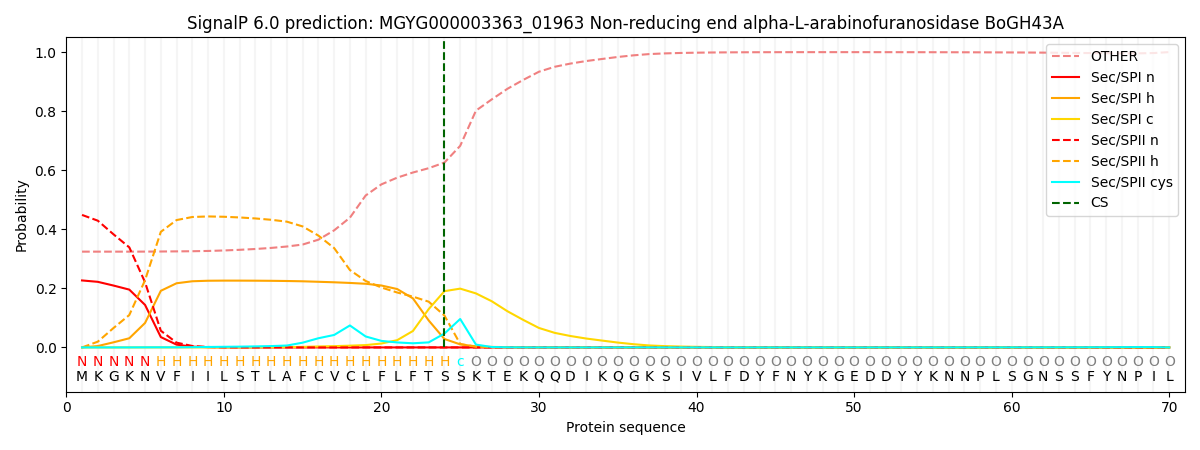
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.331659 | 0.213644 | 0.452682 | 0.000526 | 0.000454 | 0.001037 |