You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003035_00062
You are here: Home > Sequence: MGYG000003035_00062
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-485 sp900542185 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; CAG-485; CAG-485 sp900542185 | |||||||||||
| CAZyme ID | MGYG000003035_00062 | |||||||||||
| CAZy Family | GH26 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 89570; End: 90952 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH26 | 151 | 455 | 7.4e-89 | 0.9933993399339934 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam02156 | Glyco_hydro_26 | 8.06e-46 | 150 | 455 | 1 | 311 | Glycosyl hydrolase family 26. |
| COG4124 | ManB2 | 4.54e-13 | 302 | 373 | 177 | 254 | Beta-mannanase [Carbohydrate transport and metabolism]. |
| cd14948 | BACON | 6.54e-11 | 47 | 137 | 7 | 83 | Bacteroidetes-Associated Carbohydrate-binding (putative) Often N-terminal (BACON) domain. The BACON domain is found in diverse domain architectures and accociated with a wide variety of domains, including carbohydrate-active enzymes and proteases. It was named for its suggested function of carbohydrate binding; the latter was inferred from domain architectures, sequence conservation, and phyletic distribution. However, recent experimental data suggest that its primary function in Bacteroides ovatus endo-xyloglucanase BoGH5A is to distance the catalytic module from the cell surface and confer additional mobility to the catalytic domain for attack of the polysaccharide. No evidence for a direct role in carbohydrate binding could be found in that case. The large majority of BACON domains are found in Bacteroidetes. |
| pfam13004 | BACON | 1.33e-04 | 72 | 137 | 4 | 61 | Putative binding domain, N-terminal. The BACON (Bacteroidetes-Associated Carbohydrate-binding Often N-terminal) domain is an all-beta domain found in diverse architectures, principally in combination with carbohydrate-active enzymes and proteases. These architectures suggest a carbohydrate-binding function which is also supported by the nature of BACON's few conserved amino-acids. The phyletic distribution of BACON and other data tentatively suggest that it may frequently function to bind mucin. Further work with the characterized structure of a member of glycoside hydrolase family 5 enzyme, Structure 3ZMR, has found no evidence for carbohydrate-binding for this domain. |
| pfam19190 | BACON_2 | 1.53e-04 | 51 | 139 | 11 | 91 | Viral BACON domain. This family represents a distinct class of BACON domains found in crAss-like phages, the most common viral family in the human gut, in which they are found in tail fiber genes. This suggests they may play a role in phage-host interactions. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CEA16910.1 | 5.79e-81 | 141 | 460 | 243 | 558 |
| QGY44077.1 | 1.54e-79 | 144 | 460 | 131 | 461 |
| SCD21902.1 | 2.56e-79 | 141 | 460 | 288 | 603 |
| QIK58757.1 | 6.87e-76 | 49 | 460 | 52 | 456 |
| ARK08557.1 | 3.78e-75 | 114 | 460 | 100 | 456 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6HF2_A | 2.00e-75 | 154 | 425 | 50 | 314 | Thestructure of BoMan26B, a GH26 beta-mannanase from Bacteroides ovatus [Bacteroides ovatus ATCC 8483],6HF4_A The structure of BoMan26B, a GH26 beta-mannanase from Bacteroides ovatus, complexed with G1M4 [Bacteroides ovatus ATCC 8483] |
| 6D2X_A | 1.56e-64 | 152 | 456 | 7 | 334 | Crystalstructure of the GH26 domain from PbGH26-GH5A endo-beta-mannanase/endo-beta-glucanase from Prevotella bryantii [Prevotella bryantii B14] |
| 6D2W_A | 1.13e-60 | 152 | 456 | 98 | 425 | Crystalstructure of Prevotella bryantii endo-beta-mannanase/endo-beta-glucanase PbGH26A-GH5A [Prevotella bryantii B14],6D2W_B Crystal structure of Prevotella bryantii endo-beta-mannanase/endo-beta-glucanase PbGH26A-GH5A [Prevotella bryantii B14] |
| 3WDQ_A | 3.25e-56 | 142 | 457 | 27 | 353 | Crystalstructure of beta-mannanase from a symbiotic protist of the termite Reticulitermes speratus [Symbiotic protist of Reticulitermes speratus],3WDR_A Crystal structure of beta-mannanase from a symbiotic protist of the termite Reticulitermes speratus complexed with gluco-manno-oligosaccharide [Symbiotic protist of Reticulitermes speratus] |
| 6HPF_A | 1.62e-54 | 148 | 456 | 5 | 310 | Structureof Inactive E165Q mutant of fungal non-CBM carrying GH26 endo-b-mannanase from Yunnania penicillata in complex with alpha-62-61-di-galactosyl-mannotriose [Yunnania penicillata] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P49425 | 4.10e-56 | 152 | 458 | 149 | 459 | Mannan endo-1,4-beta-mannosidase OS=Rhodothermus marinus (strain ATCC 43812 / DSM 4252 / R-10) OX=518766 GN=manA PE=1 SV=3 |
| G2Q4H7 | 1.47e-46 | 128 | 456 | 153 | 476 | Mannan endo-1,4-beta-mannosidase OS=Myceliophthora thermophila (strain ATCC 42464 / BCRC 31852 / DSM 1799) OX=573729 GN=Man26A PE=1 SV=1 |
| Q5AWB7 | 1.02e-45 | 175 | 456 | 50 | 350 | Probable mannan endo-1,4-beta-mannosidase E OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=manE PE=3 SV=1 |
| P55296 | 7.00e-45 | 154 | 457 | 162 | 461 | Mannan endo-1,4-beta-mannosidase A OS=Piromyces sp. OX=45796 GN=MANA PE=2 SV=1 |
| P55298 | 8.16e-45 | 154 | 457 | 161 | 460 | Mannan endo-1,4-beta-mannosidase C OS=Piromyces sp. OX=45796 GN=MANC PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
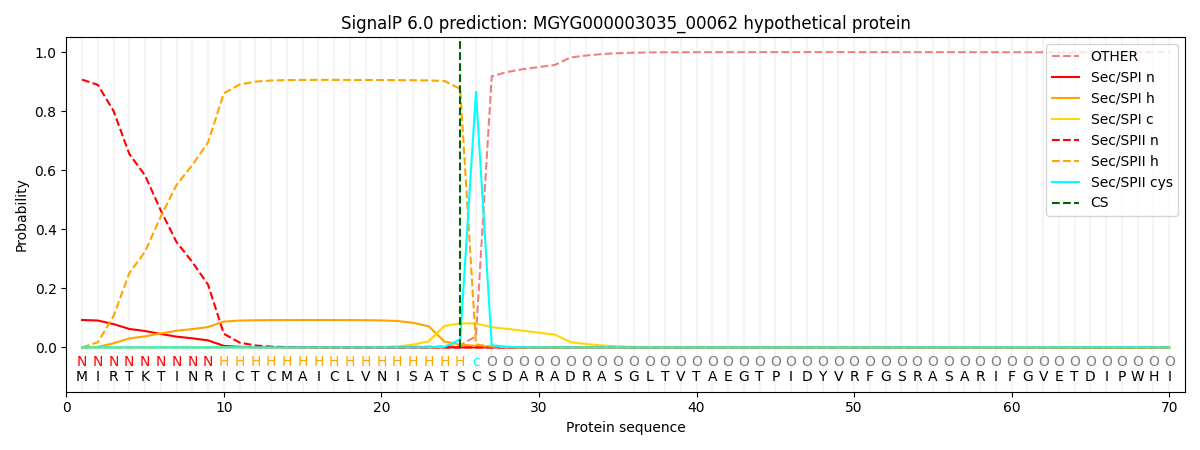
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000390 | 0.090068 | 0.909267 | 0.000117 | 0.000087 | 0.000070 |
