You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002476_03432
You are here: Home > Sequence: MGYG000002476_03432
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Yersinia pestis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Enterobacteriaceae; Yersinia; Yersinia pestis | |||||||||||
| CAZyme ID | MGYG000002476_03432 | |||||||||||
| CAZy Family | AA2 | |||||||||||
| CAZyme Description | Catalase-peroxidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 3662557; End: 3664770 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| AA2 | 86 | 272 | 4.8e-22 | 0.5764705882352941 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd00649 | catalase_peroxidase_1 | 0.0 | 25 | 430 | 1 | 409 | N-terminal catalytic domain of catalase-peroxidases. This is a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Catalase-peroxidases can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. These enzymes are found in many archaeal and bacterial organisms, where they neutralize potentially lethal hydrogen peroxide molecules generated during photosynthesis or stationary phase. Along with related intracellular fungal and plant peroxidases, catalase-peroxidases belong to class I of the plant peroxidase superfamily. Unlike the eukaryotic enzymes, they are typically comprised of two homologous domains that probably arose via a single gene duplication event. The heme binding motif is present only in the N-terminal domain; the function of the C-terminal domain is not clear. |
| cd08200 | catalase_peroxidase_2 | 0.0 | 434 | 731 | 1 | 297 | C-terminal non-catalytic domain of catalase-peroxidases. This is a subgroup of heme-dependent peroxidases of the plant superfamily that share a heme prosthetic group and catalyze a multistep oxidative reaction involving hydrogen peroxide as the electron acceptor. Catalase-peroxidases can exhibit both catalase and broad-spectrum peroxidase activities depending on the steady-state concentration of hydrogen peroxide. These enzymes are found in many archaeal and bacterial organisms where they neutralize potentially lethal hydrogen peroxide molecules generated during photosynthesis or stationary phase. Along with related intracellular fungal and plant peroxidases, catalase-peroxidases belong to plant peroxidase superfamily. Unlike the eukaryotic enzymes, they are typically comprised of two homologous domains that probably arose via a single gene duplication event. The heme binding motif is present only in the N-terminal domain; the function of the C-terminal domain is not clear. |
| COG0376 | KatG | 0.0 | 1 | 735 | 1 | 730 | Catalase (peroxidase I) [Inorganic ion transport and metabolism]. |
| TIGR00198 | cat_per_HPI | 0.0 | 16 | 734 | 2 | 713 | catalase/peroxidase HPI. As catalase, this enzyme catalyzes the dismutation of two molecules of hydrogen peroxide to dioxygen and two molecules of water. As a peroxidase, it uses hydrogen peroxide to oxidize donor compounds and produce water. KatG from E. coli is a homotetramer with two non-covalently associated iron protoheme IX groups per tetramer, but the ortholog from Synechococcus sp. is a homodimer with one protoheme. Important sites (numbered according to E. coli KatG) include heme ligands His-106 and His-267 and active site Trp-318. Note that the translation PID:g296476 from accession X71420 from Rhodobacter capsulatus B10 contains extensive frameshift differences from the rest of the orthologous family. [Cellular processes, Detoxification] |
| PRK15061 | PRK15061 | 0.0 | 19 | 735 | 7 | 726 | catalase/peroxidase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QZY04649.1 | 7.58e-295 | 28 | 735 | 3 | 715 |
| QPC71243.1 | 1.58e-277 | 33 | 737 | 61 | 776 |
| BCS01357.1 | 8.89e-31 | 67 | 409 | 6 | 264 |
| BCS13101.1 | 8.89e-31 | 67 | 409 | 6 | 264 |
| QQK46267.1 | 2.48e-30 | 65 | 409 | 3 | 263 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5L05_A | 0.0 | 19 | 734 | 8 | 727 | Crystalstructure of catalase-peroxidase KATG of burkholderia pseudomallei treated with INH [Burkholderia pseudomallei 1710b],5L05_B Crystal structure of catalase-peroxidase KATG of burkholderia pseudomallei treated with INH [Burkholderia pseudomallei 1710b],5SW6_A Crystal structure of an oxoferryl species of catalase-peroxidase KatG at pH5.6 [Burkholderia pseudomallei 1710b],5SW6_B Crystal structure of an oxoferryl species of catalase-peroxidase KatG at pH5.6 [Burkholderia pseudomallei 1710b],5SX0_B Crystal structure of an oxoferryl species of catalase-peroxidase KatG at pH7.5 [Burkholderia pseudomallei 1710b],5SX3_A Crystal structure of the catalase-peroxidase KatG of B. pseudomaallei at pH 4.5 [Burkholderia pseudomallei 1710b],5SX3_B Crystal structure of the catalase-peroxidase KatG of B. pseudomaallei at pH 4.5 [Burkholderia pseudomallei 1710b],5SXQ_A Crystal structure of B. pseudomallei KatG with isonicotinic acid hydrazide bound [Burkholderia pseudomallei 1710b],5SXQ_B Crystal structure of B. pseudomallei KatG with isonicotinic acid hydrazide bound [Burkholderia pseudomallei 1710b],5SXS_A Crystal structure of catalase-peroxidase KatG with isonicotinic acid hydrazide and AMP bound [Burkholderia pseudomallei 1710b],5SXS_B Crystal structure of catalase-peroxidase KatG with isonicotinic acid hydrazide and AMP bound [Burkholderia pseudomallei 1710b],5SYL_A B. pseudomallei KatG with KCN bound [Burkholderia pseudomallei 1710b],5SYL_B B. pseudomallei KatG with KCN bound [Burkholderia pseudomallei 1710b],6MPY_A B. pseudomallei KatG crystallized in the presence of benzoyl hydrazide [Burkholderia pseudomallei],6MPY_B B. pseudomallei KatG crystallized in the presence of benzoyl hydrazide [Burkholderia pseudomallei],6MQ0_A B. pseudomallei KatG crystallized in the presence of furoyl hydrazide [Burkholderia pseudomallei],6MQ0_B B. pseudomallei KatG crystallized in the presence of furoyl hydrazide [Burkholderia pseudomallei],6MQ1_A Chain A, Catalase-peroxidase [Burkholderia pseudomallei],6MQ1_B Chain B, Catalase-peroxidase [Burkholderia pseudomallei] |
| 5SX0_A | 0.0 | 19 | 734 | 8 | 727 | Crystalstructure of an oxoferryl species of catalase-peroxidase KatG at pH7.5 [Burkholderia pseudomallei 1710b] |
| 5SX1_A | 2.54e-319 | 19 | 734 | 8 | 727 | Crystalstructure of D141E variant of B. pseudomallei KatG [Burkholderia pseudomallei 1710b],5SX1_B Crystal structure of D141E variant of B. pseudomallei KatG [Burkholderia pseudomallei 1710b] |
| 5KSN_A | 2.54e-319 | 19 | 734 | 8 | 727 | Crystalstructure of the S324G variant of catalase-peroxidase from B. pseudomallei with INH bound [Burkholderia pseudomallei 1710b],5KSN_B Crystal structure of the S324G variant of catalase-peroxidase from B. pseudomallei with INH bound [Burkholderia pseudomallei 1710b] |
| 5SXT_A | 2.54e-319 | 19 | 734 | 8 | 727 | Crystalstructure of the S324T variant of Burkholderia pseudomallei KatG with isonicotinic acid hydrazide bound [Burkholderia pseudomallei 1710b],5SXT_B Crystal structure of the S324T variant of Burkholderia pseudomallei KatG with isonicotinic acid hydrazide bound [Burkholderia pseudomallei 1710b] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q0HZ78 | 0.0 | 1 | 734 | 1 | 739 | Catalase-peroxidase 2 OS=Shewanella sp. (strain MR-7) OX=60481 GN=katG2 PE=3 SV=1 |
| Q5WZY1 | 0.0 | 1 | 735 | 1 | 741 | Catalase-peroxidase 2 OS=Legionella pneumophila (strain Lens) OX=297245 GN=katG2 PE=3 SV=1 |
| Q1CLM4 | 0.0 | 1 | 737 | 1 | 737 | Catalase-peroxidase OS=Yersinia pestis bv. Antiqua (strain Nepal516) OX=377628 GN=katG PE=3 SV=1 |
| Q1C435 | 0.0 | 1 | 737 | 1 | 737 | Catalase-peroxidase OS=Yersinia pestis bv. Antiqua (strain Antiqua) OX=360102 GN=katG PE=3 SV=1 |
| Q5X8J8 | 0.0 | 1 | 735 | 1 | 741 | Catalase-peroxidase 2 OS=Legionella pneumophila (strain Paris) OX=297246 GN=katG2 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
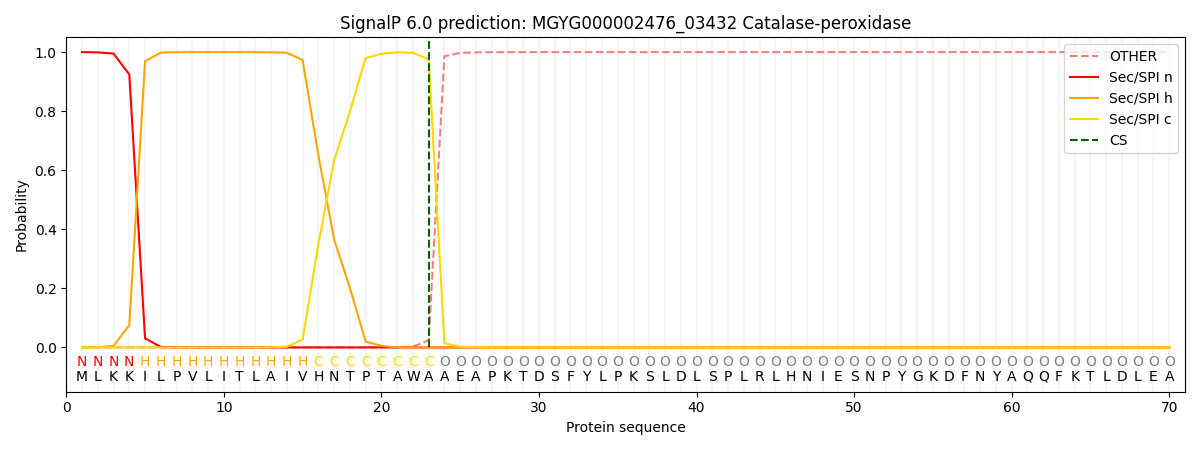
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000399 | 0.998739 | 0.000210 | 0.000210 | 0.000207 | 0.000178 |
