You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002230_01697
You are here: Home > Sequence: MGYG000002230_01697
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
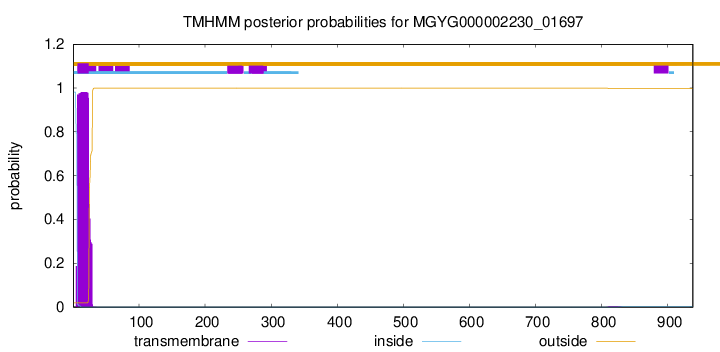
TMHMM annotations
Basic Information help
| Species | Ruminococcus sp900545125 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus; Ruminococcus sp900545125 | |||||||||||
| CAZyme ID | MGYG000002230_01697 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1507; End: 4323 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 264 | 448 | 7.9e-53 | 0.9076923076923077 |
| CBM13 | 562 | 704 | 1.4e-22 | 0.6968085106382979 |
| CBM13 | 715 | 874 | 2.9e-18 | 0.7872340425531915 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 4.22e-40 | 174 | 511 | 17 | 332 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam00544 | Pec_lyase_C | 1.08e-20 | 262 | 445 | 30 | 208 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
| pfam14200 | RicinB_lectin_2 | 1.32e-20 | 645 | 743 | 1 | 89 | Ricin-type beta-trefoil lectin domain-like. |
| smart00656 | Amb_all | 1.61e-20 | 260 | 448 | 10 | 186 | Amb_all domain. |
| cd14256 | Dockerin_I | 5.41e-17 | 881 | 936 | 1 | 56 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBL16867.1 | 0.0 | 35 | 934 | 33 | 910 |
| CDM68184.1 | 2.30e-110 | 39 | 618 | 30 | 584 |
| AYC52826.1 | 3.41e-93 | 35 | 521 | 28 | 479 |
| AOP16278.1 | 3.41e-93 | 35 | 521 | 28 | 479 |
| QAW38679.1 | 3.41e-93 | 35 | 521 | 28 | 479 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3ZSC_A | 2.08e-11 | 200 | 425 | 14 | 212 | Catalyticfunction and substrate recognition of the pectate lyase from Thermotoga maritima [Thermotoga maritima] |
| 5B2H_A | 1.38e-06 | 629 | 755 | 163 | 280 | Crystalstructure of HA33 from Clostridium botulinum serotype C strain Yoichi [Clostridium botulinum],5B2H_B Crystal structure of HA33 from Clostridium botulinum serotype C strain Yoichi [Clostridium botulinum] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P94449 | 5.61e-61 | 185 | 522 | 33 | 338 | Pectin lyase OS=Bacillus subtilis OX=1423 GN=pelB PE=1 SV=1 |
| O34819 | 3.80e-60 | 185 | 522 | 33 | 338 | Pectin lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pelB PE=3 SV=1 |
| P27027 | 5.28e-50 | 187 | 521 | 10 | 304 | Pectin lyase OS=Pseudomonas marginalis OX=298 GN=pnl PE=1 SV=2 |
| P24112 | 1.03e-42 | 201 | 523 | 13 | 308 | Pectin lyase OS=Pectobacterium carotovorum OX=554 GN=pnl PE=1 SV=1 |
| Q00645 | 3.63e-15 | 193 | 425 | 39 | 234 | Pectate lyase plyB OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=plyB PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001770 | 0.237357 | 0.760412 | 0.000140 | 0.000156 | 0.000145 |