You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001378_03387
You are here: Home > Sequence: MGYG000001378_03387
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides ovatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides ovatus | |||||||||||
| CAZyme ID | MGYG000001378_03387 | |||||||||||
| CAZy Family | GH33 | |||||||||||
| CAZyme Description | Hercynine oxygenase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 180055; End: 182046 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 312 | 610 | 1.4e-18 | 0.8304093567251462 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam03781 | FGE-sulfatase | 7.89e-50 | 28 | 248 | 5 | 258 | Sulfatase-modifying factor enzyme 1. This domain is found in eukaryotic proteins required for post-translational sulfatase modification (SUMF1). These proteins are associated with the rare disorder multiple sulfatase deficiency (MSD). The protein product of the SUMF1 gene is FGE, formylglycine (FGly),-generating enzyme, which is a sulfatase. Sulfatases are enzymes essential for degradation and remodelling of sulfate esters, and formylglycine (FGly), the key catalytic in the active site, is unique to sulfatases. FGE is localized to the endoplasmic reticulum (ER) and interacts with and modifies the unfolded form of newly synthesized sulfatases. FGE is a single-domain monomer with a surprising paucity of secondary structure that adopts a unique fold which is stabilized by two Ca2+ ions. The effect of all mutations found in MSD patients is explained by the FGE structure, providing a molecular basis for MSD. A redox-active disulfide bond is present in the active site of FGE. An oxidized cysteine residue, possibly cysteine sulfenic acid, has been detected that may allow formulation of a structure-based mechanism for FGly formation from cysteine residues in all sulfatases. In Mycobacteria and Treponema denticola this enzyme functions as an iron(II)-dependent oxidoreductase. |
| COG1262 | YfmG | 2.21e-47 | 14 | 250 | 39 | 312 | Formylglycine-generating enzyme, required for sulfatase activity, contains SUMF1/FGE domain [Posttranslational modification, protein turnover, chaperones]. |
| cd15482 | Sialidase_non-viral | 4.48e-33 | 300 | 648 | 5 | 321 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| pfam13088 | BNR_2 | 6.38e-21 | 320 | 609 | 1 | 261 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
| COG4692 | COG4692 | 1.23e-07 | 284 | 662 | 28 | 377 | Predicted neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QGT74043.1 | 0.0 | 1 | 663 | 1 | 663 |
| QNL36993.1 | 0.0 | 1 | 663 | 1 | 663 |
| SCV08950.1 | 0.0 | 1 | 663 | 1 | 663 |
| ALJ49526.1 | 0.0 | 1 | 663 | 1 | 663 |
| QRQ56304.1 | 0.0 | 1 | 663 | 1 | 663 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5HHA_A | 8.87e-30 | 28 | 247 | 42 | 282 | Structureof PvdO from Pseudomonas aeruginosa [Pseudomonas aeruginosa PAO1],5HHA_B Structure of PvdO from Pseudomonas aeruginosa [Pseudomonas aeruginosa PAO1] |
| 1Y1F_X | 3.52e-25 | 18 | 246 | 9 | 292 | humanformylglycine generating enzyme with cysteine sulfenic acid [Homo sapiens],1Y1J_X human formylglycine generating enzyme, sulfonic acid/desulfurated form [Homo sapiens] |
| 1Y1E_X | 3.52e-25 | 18 | 246 | 9 | 292 | humanformylglycine generating enzyme [Homo sapiens],1Y1H_X human formylglycine generating enzyme, oxidised Cys refined as hydroperoxide [Homo sapiens],1Y1I_X hyuman formylglycine generating enzyme, reduced form [Homo sapiens] |
| 1Y1G_X | 3.52e-25 | 18 | 246 | 9 | 292 | Humanformylglycine generating enzyme, double sulfonic acid form [Homo sapiens],1Z70_X 1.15A resolution structure of the formylglycine generating enzyme FGE [Homo sapiens] |
| 2AFT_X | 3.99e-25 | 28 | 246 | 6 | 279 | ChainX, Sulfatase modifying factor 1 [Homo sapiens],2AIJ_X Chain X, Sulfatase modifying factor 1 [Homo sapiens],2AIK_X Chain X, Sulfatase modifying factor 1 [Homo sapiens],2HI8_X Chain X, Sulfatase-modifying factor 1 [Homo sapiens],2HIB_X Chain X, Sulfatase-modifying factor 1 [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q0P5L5 | 2.99e-24 | 28 | 246 | 91 | 364 | Formylglycine-generating enzyme OS=Bos taurus OX=9913 GN=SUMF1 PE=2 SV=1 |
| Q8R0F3 | 5.28e-24 | 28 | 246 | 89 | 362 | Formylglycine-generating enzyme OS=Mus musculus OX=10090 GN=Sumf1 PE=1 SV=2 |
| Q8NBK3 | 5.44e-24 | 18 | 246 | 81 | 364 | Formylglycine-generating enzyme OS=Homo sapiens OX=9606 GN=SUMF1 PE=1 SV=3 |
| Q9F3C7 | 3.45e-22 | 18 | 246 | 16 | 300 | Formylglycine-generating enzyme OS=Streptomyces coelicolor (strain ATCC BAA-471 / A3(2) / M145) OX=100226 GN=SCO7548 PE=1 SV=1 |
| Q7AJA5 | 8.97e-22 | 28 | 246 | 385 | 613 | Serine/threonine-protein kinase pkn1 OS=Chlamydia pneumoniae OX=83558 GN=pkn1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
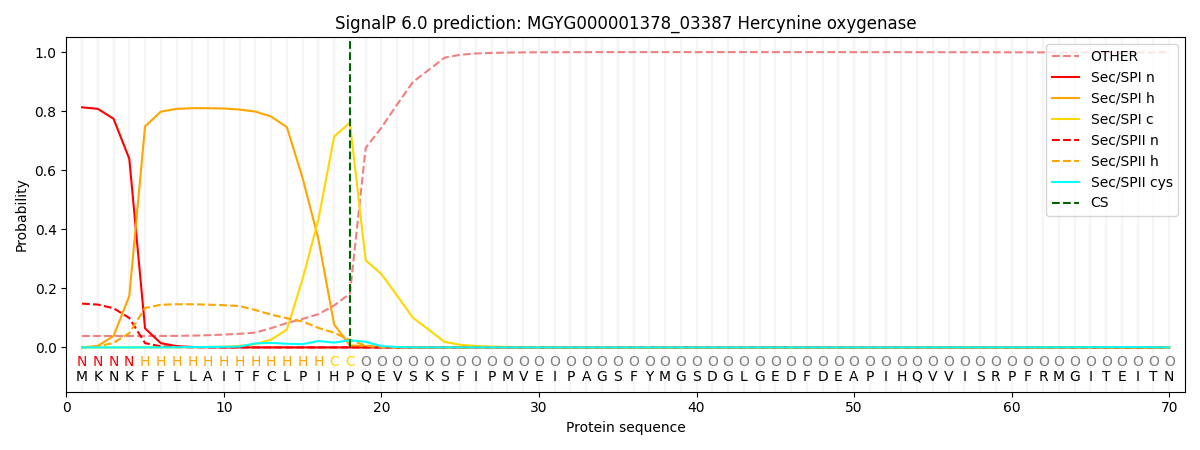
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.044494 | 0.799040 | 0.155346 | 0.000392 | 0.000342 | 0.000357 |
