You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001306_03139
You are here: Home > Sequence: MGYG000001306_03139
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phocaeicola coprocola | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Phocaeicola; Phocaeicola coprocola | |||||||||||
| CAZyme ID | MGYG000001306_03139 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | Non-reducing end alpha-L-arabinofuranosidase BoGH43B | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 28778; End: 29473 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 42 | 231 | 2.5e-65 | 0.7380073800738007 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd09001 | GH43_FsAxh1-like | 2.50e-90 | 39 | 231 | 1 | 197 | Glycosyl hydrolase family 43 such as Fibrobacter succinogenes subsp. succinogenes S85 arabinoxylan alpha-L-arabinofuranosidase. This glycosyl hydrolase family 43 (GH43) includes mostly enzymes that have been annotated as having beta-1,4-xylosidase (beta-D-xylosidase; xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity. They are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. This subfamily includes the characterized Clostridium stercorarium F-9 beta-xylosidase Xyl43B. It also includes Humicola insolens AXHd3 (HiAXHd3), a GH43 arabinofuranosidase (EC 3.2.1.55) that hydrolyzes O3-linked arabinose of doubly substituted xylans, a feature of the polysaccharide that is recalcitrant to degradation. It possesses an additional C-terminal beta-sandwich domain such that the interface between the domains comprises a xylan binding cleft that houses the active site pocket. The HiAXHd3 active site is tuned to hydrolyze arabinofuranosyl or xylosyl linkages, and the topology of the distal regions of the substrate binding surface confers specificity. It also includes Fibrobacter succinogenes subsp. succinogenes S85 arabinoxylan alpha-L-arabinofuranosidase (Axh1;Fisuc_1769;FSU_2269), Paenibacillus sp. E18 alpha-L-arabinofuranosidase (Abf43A), Bifidobacterium adolescentis ATCC 15703 double substituted xylan alpha-1,3-L-specific arabinofuranosidase d3 (AXHd3;AXH-d3;BaAXH-d3;BAD_0301;E-AFAM2), and Chrysosporium lucknowense C1 arabinoxylan hydrolase / double substituted xylan alpha-1,3-L-arabinofuranosidase (Abn7;AXHd). A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| pfam04616 | Glyco_hydro_43 | 2.04e-48 | 40 | 231 | 1 | 200 | Glycosyl hydrolases family 43. The glycosyl hydrolase family 43 contains members that are arabinanases. Arabinanases hydrolyze the alpha-1,5-linked L-arabinofuranoside backbone of plant cell wall arabinans. The structure of arabinanase Arb43A from Cellvibrio japonicus reveals a five-bladed beta-propeller fold. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd18617 | GH43_XynB-like | 2.95e-42 | 42 | 230 | 1 | 200 | Glycosyl hydrolase family 43, such as Bacteroides ovatus alpha-L-arabinofuranosidase (BoGH43, XynB). This glycosyl hydrolase family 43 (GH43) subgroup includes enzymes that have been characterized to have alpha-L-arabinofuranosidase (EC 3.2.1.55) and beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activities. Beta-1,4-xylosidases are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Also included in this subfamily are Bacteroides ovatus alpha-L-arabinofuranosidases, BoGH43A and BoGH43B, both having a two-domain architecture, consisting of an N-terminal 5-bladed beta-propeller domain harboring the catalytic active site, and a C-terminal beta-sandwich domain. However, despite significant functional overlap between these two enzymes, BoGH43A and BoGH43B share just 41% sequence identity. The latter appears to be significantly less active on the same substrates, suggesting that these paralogs may play subtly different roles during the degradation of xyloglucans from different sources, or may function most optimally at different stages in the catabolism of xyloglucan oligosaccharides (XyGOs), for example before or after hydrolysis of certain side-chain moieties. It also includes Phanerochaete chrysosporium BKM-F-1767 Xyl, a bifunctional xylosidase/arabinofuranosidase. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08989 | GH43_XYL-like | 8.54e-41 | 42 | 226 | 1 | 190 | Glycosyl hydrolase family 43, beta-D-xylosidases and arabinofuranosidases. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes that have been annotated as having beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity, including Selenomonas ruminantium beta-D-xylosidase SXA. These are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. It also includes various GH43 family GH43 arabinofuranosidases (EC 3.2.1.55) including Humicola insolens alpha-L-arabinofuranosidase AXHd3, Bacteroides ovatus alpha-L-arabinofuranosidase (BoGH43, XynB), and the bifunctional Phanerochaete chrysosporium xylosidase/arabinofuranosidase (Xyl;PcXyl). GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd09002 | GH43_XYL-like | 2.08e-35 | 40 | 226 | 1 | 182 | Glycosyl hydrolase family 43, beta-D-xylosidase (uncharacterized). This glycosyl hydrolase family 43 (GH43) subgroup includes enzymes that have been annotated as having beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity. They are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJR62592.1 | 1.77e-127 | 1 | 231 | 1 | 226 |
| QJR75289.1 | 1.77e-127 | 1 | 231 | 1 | 226 |
| AII65237.1 | 1.77e-127 | 1 | 231 | 1 | 226 |
| QJR71190.1 | 1.77e-127 | 1 | 231 | 1 | 226 |
| QJR66850.1 | 1.77e-127 | 1 | 231 | 1 | 226 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2EXH_A | 1.23e-26 | 42 | 226 | 6 | 206 | Structureof the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_B Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_C Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus],2EXH_D Structure of the family43 beta-Xylosidase from geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 1YRZ_A | 1.64e-26 | 42 | 226 | 7 | 207 | ChainA, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125],1YRZ_B Chain B, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125] |
| 5JOZ_A | 2.89e-26 | 39 | 226 | 4 | 195 | Bacteroidesovatus Xyloglucan PUL GH43B [Bacteroides ovatus],5JOZ_B Bacteroides ovatus Xyloglucan PUL GH43B [Bacteroides ovatus] |
| 2EXI_A | 1.10e-25 | 42 | 226 | 6 | 206 | ChainA, beta-D-xylosidase [Geobacillus stearothermophilus],2EXI_B Chain B, beta-D-xylosidase [Geobacillus stearothermophilus],2EXI_C Chain C, beta-D-xylosidase [Geobacillus stearothermophilus],2EXI_D Chain D, beta-D-xylosidase [Geobacillus stearothermophilus] |
| 2EXK_A | 1.10e-25 | 42 | 226 | 6 | 206 | ChainA, beta-D-xylosidase [Geobacillus stearothermophilus],2EXK_B Chain B, beta-D-xylosidase [Geobacillus stearothermophilus],2EXK_C Chain C, beta-D-xylosidase [Geobacillus stearothermophilus],2EXK_D Chain D, beta-D-xylosidase [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9WXE8 | 2.53e-111 | 32 | 231 | 33 | 227 | Putative beta-xylosidase OS=Prevotella ruminicola OX=839 PE=3 SV=2 |
| T2KN85 | 7.64e-48 | 31 | 224 | 42 | 251 | Beta-xylosidase OS=Formosa agariphila (strain DSM 15362 / KCTC 12365 / LMG 23005 / KMM 3901 / M-2Alg 35-1) OX=1347342 GN=BN863_22160 PE=1 SV=1 |
| A7LXU0 | 1.87e-26 | 1 | 226 | 2 | 217 | Non-reducing end alpha-L-arabinofuranosidase BoGH43B OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02656 PE=1 SV=2 |
| A9ZND1 | 1.73e-25 | 39 | 229 | 4 | 211 | Xylan 1,3-beta-xylosidase OS=Vibrio sp. OX=678 GN=xloA PE=1 SV=1 |
| P94489 | 1.05e-21 | 42 | 226 | 5 | 205 | Beta-xylosidase OS=Bacillus subtilis (strain 168) OX=224308 GN=xynB PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
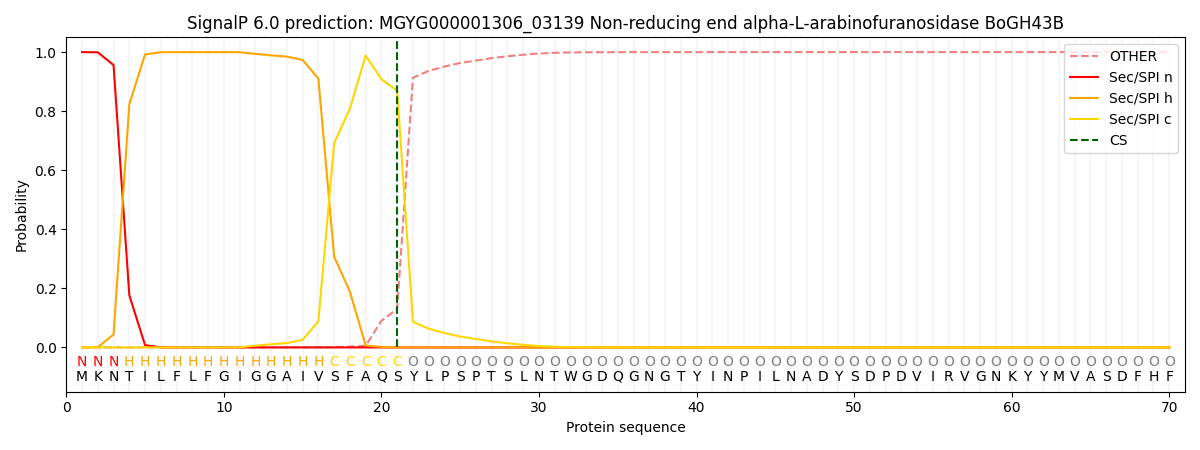
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000363 | 0.998937 | 0.000173 | 0.000169 | 0.000160 | 0.000148 |
