You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001016_00660
You are here: Home > Sequence: MGYG000001016_00660
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
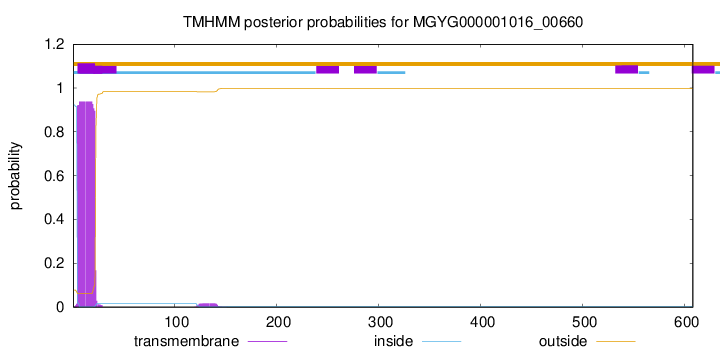
TMHMM annotations
Basic Information help
| Species | UMGS1537 sp900543695 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; UBA1212; UBA1255; UMGS1537; UMGS1537 sp900543695 | |||||||||||
| CAZyme ID | MGYG000001016_00660 | |||||||||||
| CAZy Family | GH163 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 73786; End: 75612 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH163 | 235 | 497 | 4.3e-88 | 0.9960159362549801 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam16126 | DUF4838 | 8.91e-101 | 229 | 497 | 2 | 263 | Domain of unknown function (DUF4838). This family consists of several uncharacterized proteins found in various Bacteroides and Chloroflexus species. The function of this family is unknown. |
| pfam03648 | Glyco_hydro_67N | 4.42e-04 | 46 | 139 | 16 | 118 | Glycosyl hydrolase family 67 N-terminus. Alpha-glucuronidases, components of an ensemble of enzymes central to the recycling of photosynthetic biomass, remove the alpha-1,2 linked 4-O-methyl glucuronic acid from xylans. This family represents the N-terminal region of alpha-glucuronidase. The N-terminal domain forms a two-layer sandwich, each layer being formed by a beta sheet of five strands. A further two helices form part of the interface with the central, catalytic, module (pfam07488). |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ASV73444.1 | 2.61e-115 | 3 | 604 | 2 | 585 |
| AIF26900.1 | 4.49e-112 | 38 | 548 | 5 | 522 |
| QOY90686.1 | 5.25e-109 | 39 | 567 | 19 | 531 |
| QQL44163.1 | 6.50e-101 | 38 | 567 | 12 | 531 |
| AXE18406.1 | 1.37e-96 | 19 | 600 | 280 | 838 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as LIPO

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000001 | 1.000057 | 0.000000 | 0.000000 | 0.000000 |