You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000174_00252
You are here: Home > Sequence: MGYG000000174_00252
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parabacteroides faecis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides faecis | |||||||||||
| CAZyme ID | MGYG000000174_00252 | |||||||||||
| CAZy Family | GH33 | |||||||||||
| CAZyme Description | Sialidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 305519; End: 306667 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 32 | 375 | 3.7e-107 | 0.97953216374269 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd15482 | Sialidase_non-viral | 1.52e-124 | 34 | 376 | 1 | 339 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| pfam13088 | BNR_2 | 4.31e-36 | 58 | 357 | 1 | 280 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
| pfam13859 | BNR_3 | 1.53e-17 | 57 | 252 | 8 | 193 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
| COG4409 | NanH | 1.01e-15 | 28 | 361 | 255 | 701 | Neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd00260 | Sialidase | 7.44e-11 | 36 | 199 | 3 | 157 | sialidases/neuraminidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates as well as playing roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed beta-propeller fold. This hierarchy includes eubacterial, eukaryotic, and viral sialidases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AYQ36531.1 | 2.47e-140 | 10 | 378 | 7 | 368 |
| CAZ95099.1 | 1.11e-135 | 28 | 378 | 34 | 378 |
| ASO04237.1 | 1.57e-135 | 22 | 370 | 22 | 373 |
| QEC45250.1 | 3.61e-127 | 36 | 382 | 98 | 440 |
| BBD46002.1 | 1.95e-126 | 35 | 381 | 38 | 376 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4FJ6_A | 1.17e-43 | 38 | 363 | 172 | 507 | Crystalstructure of a glycoside hydrolase family 33, candidate sialidase (BDI_2946) from Parabacteroides distasonis ATCC 8503 at 1.90 A resolution [Parabacteroides distasonis ATCC 8503],4FJ6_B Crystal structure of a glycoside hydrolase family 33, candidate sialidase (BDI_2946) from Parabacteroides distasonis ATCC 8503 at 1.90 A resolution [Parabacteroides distasonis ATCC 8503],4FJ6_C Crystal structure of a glycoside hydrolase family 33, candidate sialidase (BDI_2946) from Parabacteroides distasonis ATCC 8503 at 1.90 A resolution [Parabacteroides distasonis ATCC 8503],4FJ6_D Crystal structure of a glycoside hydrolase family 33, candidate sialidase (BDI_2946) from Parabacteroides distasonis ATCC 8503 at 1.90 A resolution [Parabacteroides distasonis ATCC 8503] |
| 6MYV_A | 1.70e-43 | 40 | 363 | 174 | 507 | Sialidase26co-crystallized with DANA-Gc [bacterium],6MYV_B Sialidase26 co-crystallized with DANA-Gc [bacterium],6MYV_C Sialidase26 co-crystallized with DANA-Gc [bacterium],6MYV_D Sialidase26 co-crystallized with DANA-Gc [bacterium] |
| 6MRV_A | 2.48e-43 | 40 | 363 | 195 | 528 | Sialidase26co-crystallized with DANA [bacterium],6MRV_B Sialidase26 co-crystallized with DANA [bacterium],6MRX_A Sialidase26 apo [bacterium],6MRX_B Sialidase26 apo [bacterium],6MRX_C Sialidase26 apo [bacterium],6MRX_D Sialidase26 apo [bacterium] |
| 6MNJ_A | 1.19e-42 | 40 | 363 | 193 | 524 | Hadzamicrobial sialidase Hz136 [Alistipes],6MNJ_B Hadza microbial sialidase Hz136 [Alistipes] |
| 2BF6_A | 9.15e-39 | 28 | 360 | 4 | 429 | AtomicResolution Structure of the bacterial sialidase NanI from Clostridium perfringens in complex with alpha-Sialic Acid (Neu5Ac). [Clostridium perfringens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O35657 | 9.15e-41 | 47 | 375 | 71 | 403 | Sialidase-1 OS=Mus musculus OX=10090 GN=Neu1 PE=1 SV=1 |
| Q99PW3 | 3.44e-40 | 47 | 375 | 71 | 403 | Sialidase-1 OS=Rattus norvegicus OX=10116 GN=Neu1 PE=1 SV=1 |
| P31206 | 7.47e-39 | 40 | 363 | 196 | 529 | Sialidase OS=Bacteroides fragilis (strain YCH46) OX=295405 GN=nanH PE=3 SV=2 |
| Q99519 | 7.50e-38 | 47 | 375 | 77 | 409 | Sialidase-1 OS=Homo sapiens OX=9606 GN=NEU1 PE=1 SV=1 |
| Q5RAF4 | 7.50e-38 | 47 | 375 | 77 | 409 | Sialidase-1 OS=Pongo abelii OX=9601 GN=NEU1 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
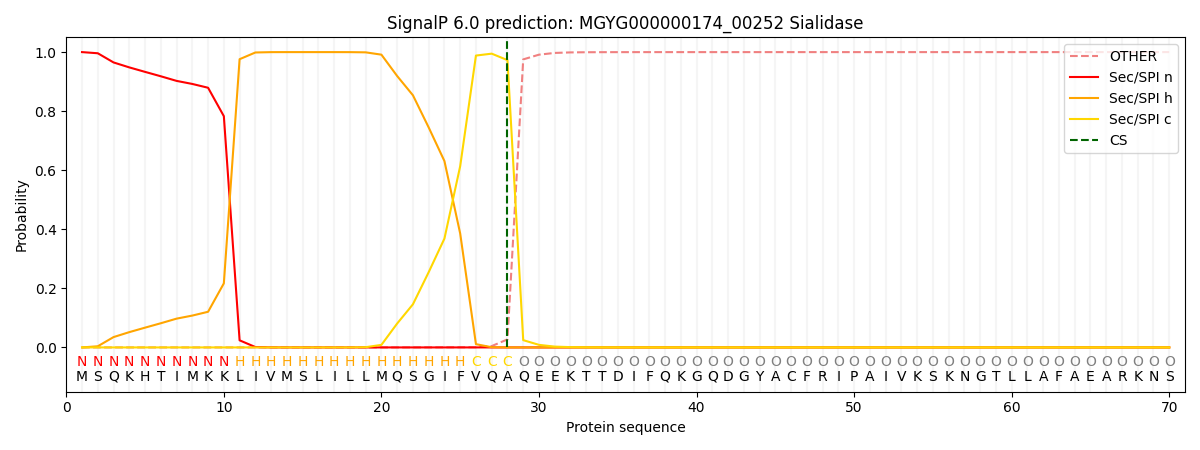
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000264 | 0.999066 | 0.000207 | 0.000148 | 0.000139 | 0.000132 |
