You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000105_01270
You are here: Home > Sequence: MGYG000000105_01270
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides clarus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides clarus | |||||||||||
| CAZyme ID | MGYG000000105_01270 | |||||||||||
| CAZy Family | GH67 | |||||||||||
| CAZyme Description | Extracellular xylan exo-alpha-(1->2)-glucuronosidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 464080; End: 466113 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH67 | 50 | 641 | 1.1e-265 | 0.898355754857997 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3661 | AguA2 | 0.0 | 21 | 640 | 4 | 674 | Alpha-glucuronidase [Carbohydrate transport and metabolism]. |
| pfam07488 | Glyco_hydro_67M | 0.0 | 108 | 411 | 1 | 324 | Glycosyl hydrolase family 67 middle domain. Alpha-glucuronidases, components of an ensemble of enzymes central to the recycling of photosynthetic biomass, remove the alpha-1,2 linked 4-O-methyl glucuronic acid from xylans. This family represents the central catalytic domain of alpha-glucuronidase. |
| pfam07477 | Glyco_hydro_67C | 2.82e-133 | 413 | 646 | 1 | 223 | Glycosyl hydrolase family 67 C-terminus. Alpha-glucuronidases, components of an ensemble of enzymes central to the recycling of photosynthetic biomass, remove the alpha-1,2 linked 4-O-methyl glucuronic acid from xylans. This family represents the C terminal region of alpha-glucuronidase which is mainly alpha-helical. It wraps around the catalytic domain (pfam07488), making additional interactions both with the N-terminal domain (pfam03648) of its parent monomer and also forming the majority of the dimer-surface with the equivalent C-terminal domain of the other monomer of the dimer. |
| pfam03648 | Glyco_hydro_67N | 2.51e-05 | 27 | 105 | 1 | 120 | Glycosyl hydrolase family 67 N-terminus. Alpha-glucuronidases, components of an ensemble of enzymes central to the recycling of photosynthetic biomass, remove the alpha-1,2 linked 4-O-methyl glucuronic acid from xylans. This family represents the N-terminal region of alpha-glucuronidase. The N-terminal domain forms a two-layer sandwich, each layer being formed by a beta sheet of five strands. A further two helices form part of the interface with the central, catalytic, module (pfam07488). |
| pfam02838 | Glyco_hydro_20b | 0.007 | 45 | 115 | 45 | 117 | Glycosyl hydrolase family 20, domain 2. This domain has a zincin-like fold. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QRQ48276.1 | 0.0 | 1 | 677 | 1 | 669 |
| QUT46117.1 | 0.0 | 1 | 677 | 26 | 694 |
| ALJ61533.1 | 0.0 | 4 | 677 | 3 | 675 |
| QUT92897.1 | 0.0 | 4 | 677 | 3 | 676 |
| QDO69417.1 | 0.0 | 4 | 677 | 3 | 676 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1GQI_A | 5.27e-239 | 22 | 653 | 1 | 683 | Structureof Pseudomonas cellulosa alpha-D-glucuronidase [Cellvibrio japonicus],1GQI_B Structure of Pseudomonas cellulosa alpha-D-glucuronidase [Cellvibrio japonicus],1GQJ_A Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with xylobiose [Cellvibrio japonicus],1GQJ_B Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with xylobiose [Cellvibrio japonicus],1GQK_A Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with glucuronic acid [Cellvibrio japonicus],1GQK_B Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with glucuronic acid [Cellvibrio japonicus],1GQL_A Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with glucuronic acid and xylotriose [Cellvibrio japonicus],1GQL_B Structure of Pseudomonas cellulosa alpha-D-glucuronidase complexed with glucuronic acid and xylotriose [Cellvibrio japonicus] |
| 1H41_A | 4.26e-238 | 22 | 653 | 1 | 683 | Pseudomonascellulosa E292A alpha-D-glucuronidase mutant complexed with aldotriuronic acid [Cellvibrio japonicus],1H41_B Pseudomonas cellulosa E292A alpha-D-glucuronidase mutant complexed with aldotriuronic acid [Cellvibrio japonicus] |
| 1MQP_A | 1.24e-173 | 74 | 639 | 86 | 669 | TheCrystal Structure Of Alpha-D-Glucuronidase From Bacillus Stearothermophilus T-6 [Geobacillus stearothermophilus] |
| 1MQR_A | 3.51e-173 | 74 | 639 | 86 | 669 | ChainA, ALPHA-D-GLUCURONIDASE [Geobacillus stearothermophilus] |
| 1K9E_A | 7.01e-173 | 74 | 639 | 86 | 669 | ChainA, alpha-D-glucuronidase [Geobacillus stearothermophilus],1K9F_A Chain A, alpha-D-glucuronidase [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| B3PC73 | 6.53e-238 | 22 | 653 | 25 | 707 | Extracellular xylan exo-alpha-(1->2)-glucuronosidase OS=Cellvibrio japonicus (strain Ueda107) OX=498211 GN=gla67A PE=1 SV=1 |
| Q09LY5 | 6.80e-173 | 74 | 639 | 86 | 669 | Xylan alpha-(1->2)-glucuronosidase OS=Geobacillus stearothermophilus OX=1422 GN=aguA PE=1 SV=1 |
| P96105 | 1.34e-162 | 66 | 640 | 75 | 665 | Xylan alpha-(1->2)-glucuronosidase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=aguA PE=1 SV=2 |
| B0Y2K1 | 1.14e-144 | 20 | 639 | 18 | 686 | Probable alpha-glucuronidase A OS=Neosartorya fumigata (strain CEA10 / CBS 144.89 / FGSC A1163) OX=451804 GN=aguA PE=3 SV=1 |
| A1CC12 | 1.61e-144 | 73 | 639 | 109 | 686 | Probable alpha-glucuronidase A OS=Aspergillus clavatus (strain ATCC 1007 / CBS 513.65 / DSM 816 / NCTC 3887 / NRRL 1 / QM 1276 / 107) OX=344612 GN=aguA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
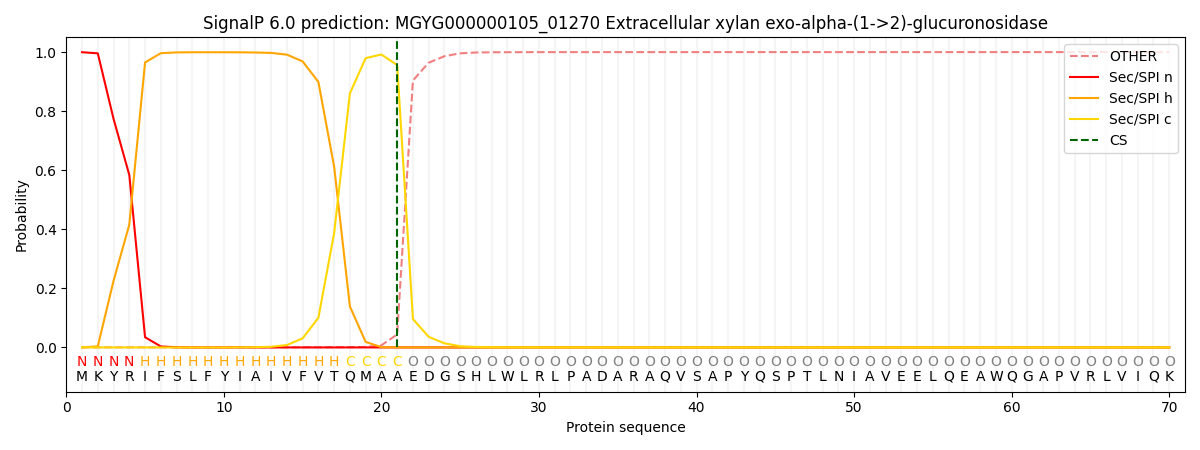
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002033 | 0.996898 | 0.000393 | 0.000226 | 0.000207 | 0.000215 |
